Introduction
The Abbreviated New Drug Application (ANDA) is a pivotal mechanism in the U.S. pharmaceutical landscape, facilitating the approval of generic drugs and ensuring patient access to affordable medications. Unlike the traditional New Drug Application (NDA), which requires extensive clinical data to establish the safety and efficacy of new drugs, the ANDA process focuses on demonstrating bioequivalence to existing brand-name drugs. This streamlined approach not only accelerates the market entry of generics but also fosters competition, ultimately driving down drug prices and enhancing public health outcomes. As the dynamics of the ANDA process evolve, particularly with the impending challenges posed by GDUFA III, understanding the intricacies of submission requirements, bioequivalence studies, and regulatory compliance becomes essential for stakeholders aiming to navigate this complex landscape successfully.
Understanding the Abbreviated New Drug Application (ANDA)
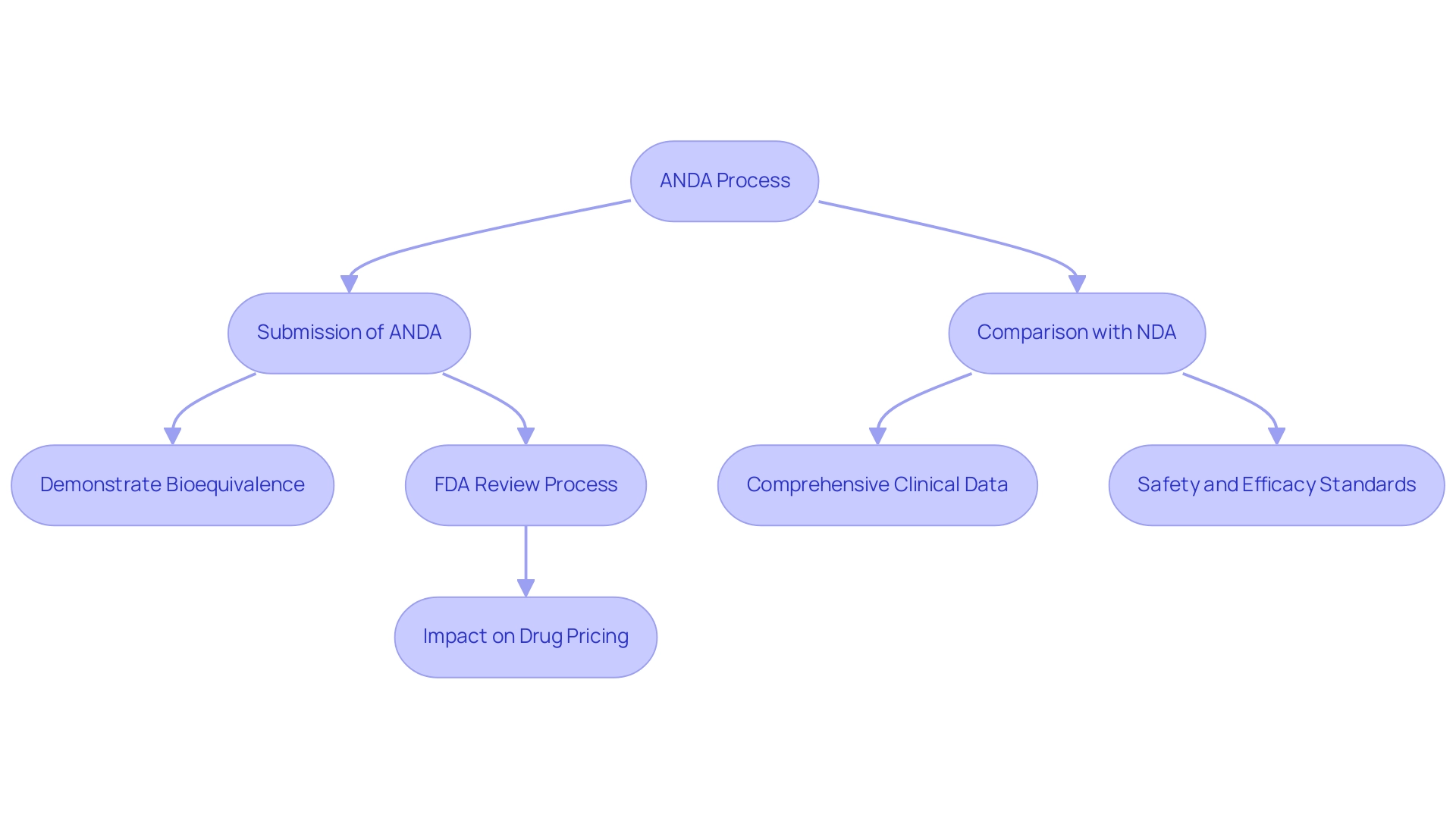
The abbreviated new medication application functions as the main route for securing FDA authorization for replica medications in the United States. Distinct from a New Drug Application (NDA), which necessitates comprehensive clinical data to substantiate the safety and efficacy of new substances, what is ANDA in pharmaceuticals refers to a process that streamlines this procedure. Its central objective is to demonstrate bioequivalence to the brand-name counterpart, ensuring that the generic medication delivers the same therapeutic effect within an equivalent timeframe.
This approach is crucial for safeguarding patient access to affordable medications while upholding stringent safety and efficacy standards. The significance of ANDAs extends beyond individual approvals; they are instrumental in enhancing competition within the pharmaceutical market. This competitive landscape can lead to decreased drug prices, thus improving accessibility for patients.
Recent trends show a shift in the FDA's review dynamics, with an annual issuance of between 4,150 and 4,500 Information Requests (IRs), including 2,480 for original applications and 1,833 for supplements. These statistics indicate evolving challenges and opportunities within the generic drug application process as GDUFA III approaches. As Bob Pollock remarked, 'Will we keep observing reduced filing rates, will generic drug approvals continue to surpass new generic applications, and what effect will that have on the old, its staffing and responsiveness?'
This highlights the ongoing complexities faced in the approval landscape.
The ANDA Submission Process: Requirements and Steps
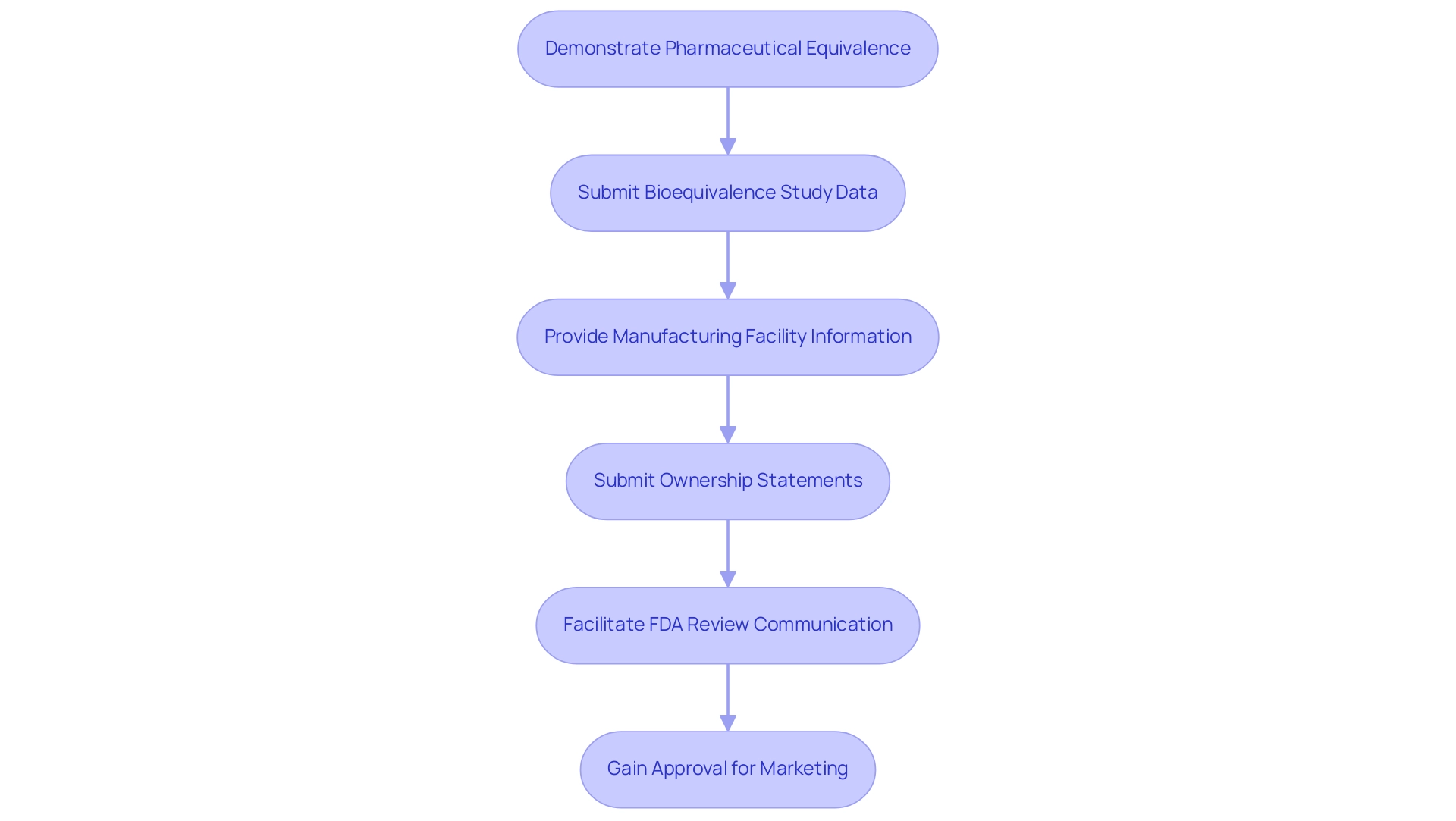
What is anda in pharmaceuticals is a vital route for introducing alternative medications to the market, and it includes several important steps that must be carefully followed to ensure adherence to FDA regulations. Initially, applicants must demonstrate that the proposed non-branded medication is pharmaceutically equivalent to the reference listed product (RLD). This entails providing detailed data on the drug's formulation, manufacturing processes, and labeling.
A comprehensive bioequivalence study must also be submitted, demonstrating that the generic product achieves the same rate and extent of absorption as the RLD. Notably, subsampling may not be appropriate for trials with fewer than 600 patients, which is a crucial consideration in bioequivalence studies. Furthermore, the application must include information regarding the manufacturing facility, confirming adherence to Good Manufacturing Practices (GMP).
Recent guidelines specify that new owners of an Abbreviated New Drug Application must either submit a statement confirming possession of a complete copy of the approved application or request one from the FDA. Clear and concise communication within the documentation package is essential for facilitating efficient review by regulatory authorities. Following submission, the FDA conducts a thorough review of the application, which may necessitate communication with the applicant for clarification or additional information.
Successful ANDA approval ultimately facilitates the marketing of the alternative medicine, which helps to illustrate what is anda in pharmaceuticals, playing a vital role in enhancing public health by providing more affordable treatment options. Richard L. Schilsky from the University of Chicago emphasizes the importance of transparency in this process, stating,
No financial relationships relevant to the content of this article have been disclosed by the independent peer reviewers.
This highlights the integrity of the review process, vital for maintaining trust in generic medication approvals.
Additionally, a case study titled 'Utilization Management Metrics' highlights how using metrics to monitor prescription medication use effectively can provide valuable insights for optimizing medication utilization and improving patient outcomes.
Key Differences Between ANDA and New Drug Application (NDA)
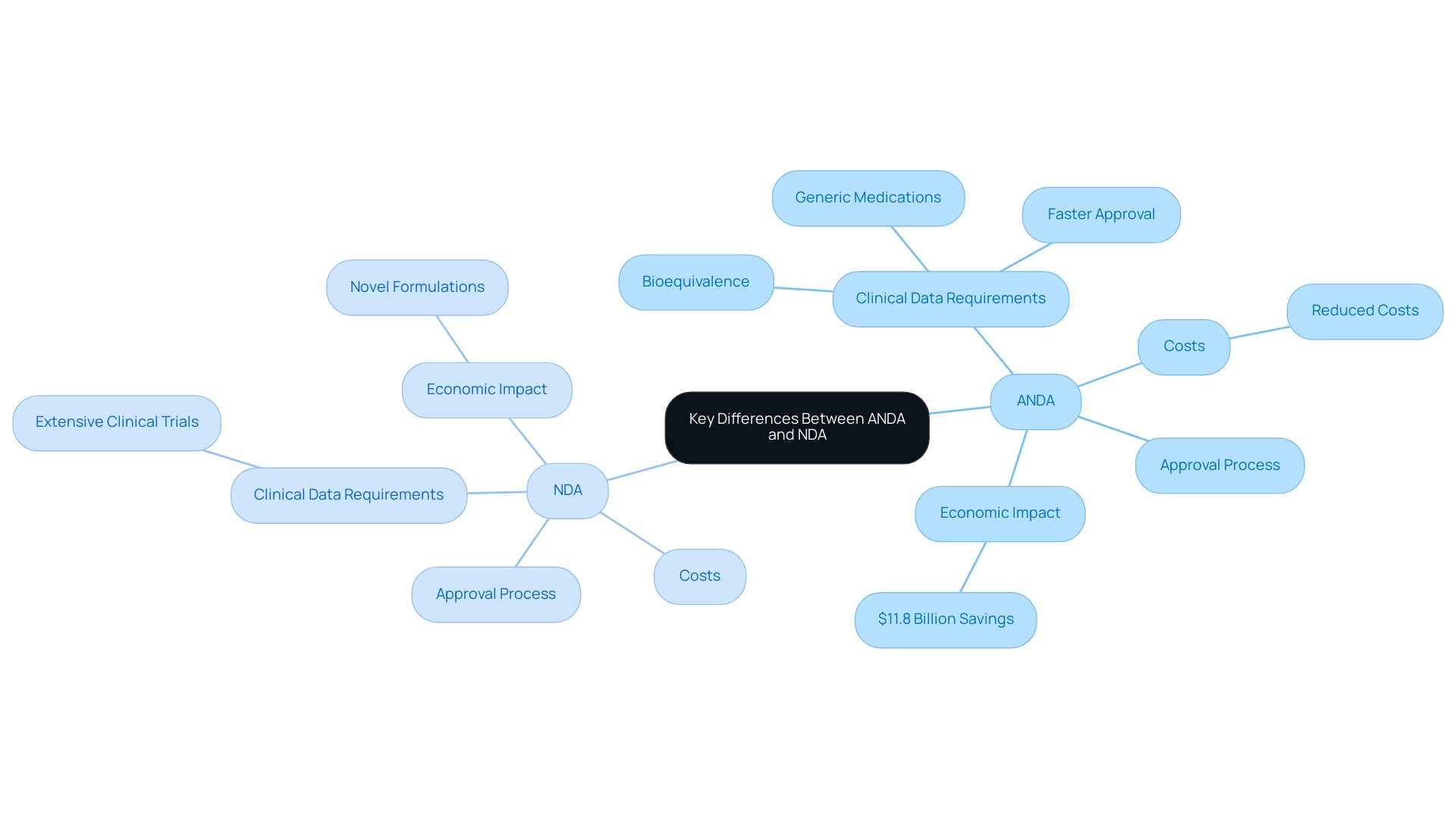
The distinction between an Abbreviated New Medication Application and a New Drug Application (NDA) is primarily rooted in the clinical data requirements. An NDA necessitates extensive clinical trials to substantiate the safety and efficacy of a new medication, while what is ANDA in pharmaceuticals focuses on establishing bioequivalence to an already approved medication. This pivotal difference leads to a quicker approval process and reduced costs for what is ANDA in pharmaceuticals, facilitating faster market entry for generic medications.
According to a study, approvals in 2017 resulted in $11.8 billion in savings to U.S. purchasers by February 2018, highlighting the economic impact of ANDA. While NDAs often relate to novel formulations that introduce new therapies, understanding what is ANDA in pharmaceuticals focuses on existing medications, which enhances competition and promotes affordability within the pharmaceutical landscape. Kathleen Uhl, Director of the FDA's Office of Non-Brand Drugs, emphasized the importance of these applications in her presentation at the Pharmaceutical Association Fall Technical Conference, stating that the FDA's prioritization of early non-brand applications is crucial for market dynamics.
However, this prioritization raises questions about its sufficiency in ensuring robust market competition, an essential consideration for stakeholders involved in pharmaceutical development and commercialization, as it influences strategic decision-making and market positioning.
The Importance of Bioequivalence Studies
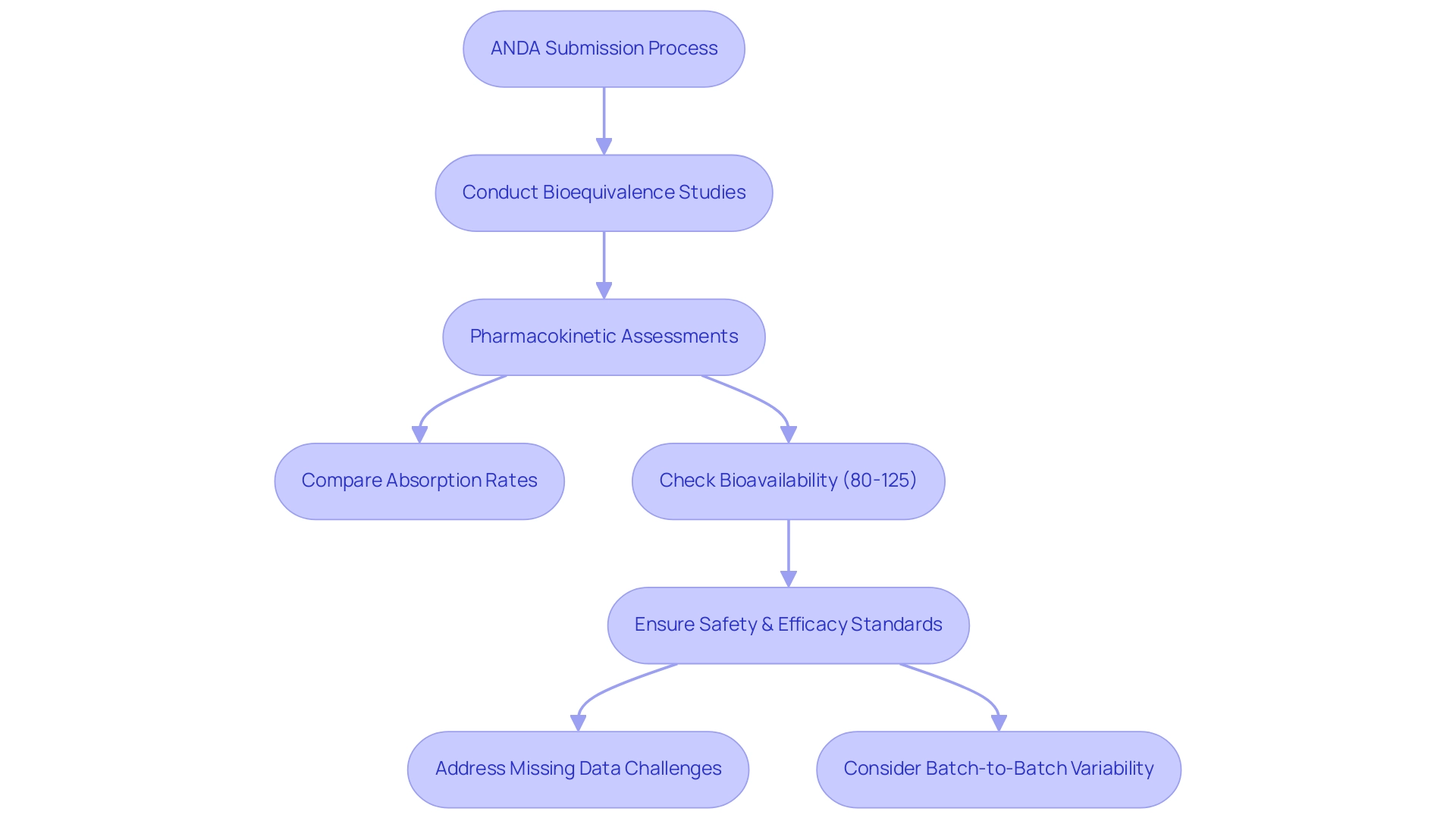
Bioequivalence studies play a crucial role in the ANDA submission process, which helps answer the question of what is ANDA in pharmaceuticals by confirming that a non-branded medication functions similarly to its branded counterpart. These studies primarily focus on pharmacokinetic assessments, which compare the rates and extents of absorption between the two medicinal formulations in healthy volunteers. For a non-branded drug to be considered bioequivalent, its bioavailability must fall within the established acceptable range of 80% to 125%, as defined by the 90% confidence interval for partial AUC.
This rigorous assessment is essential, as it ensures that patients obtain the same therapeutic benefits from the alternative formulation, thereby maintaining stringent safety and efficacy standards. The integrity of these bioequivalence studies is vital; they form the backbone of regulatory approval and foster market trust in generic medications. Recent insights from experts underscore the need for harmonization in managing missing samples, particularly for substances with long half-lives, where imputation can significantly impact statistical power.
As noted by several pharmacologists involved in the survey, 'The handling of missing data is critical, especially for long-acting drugs, to ensure robust statistical outcomes.' Furthermore, findings from a randomized clinical trial on a dry powder inhaler revealed substantial batch-to-batch pharmacokinetic variability, raising questions about the current bioequivalence regulations. Such case studies illuminate the ongoing challenges in bioequivalence assessments and their implications for regulatory standards.
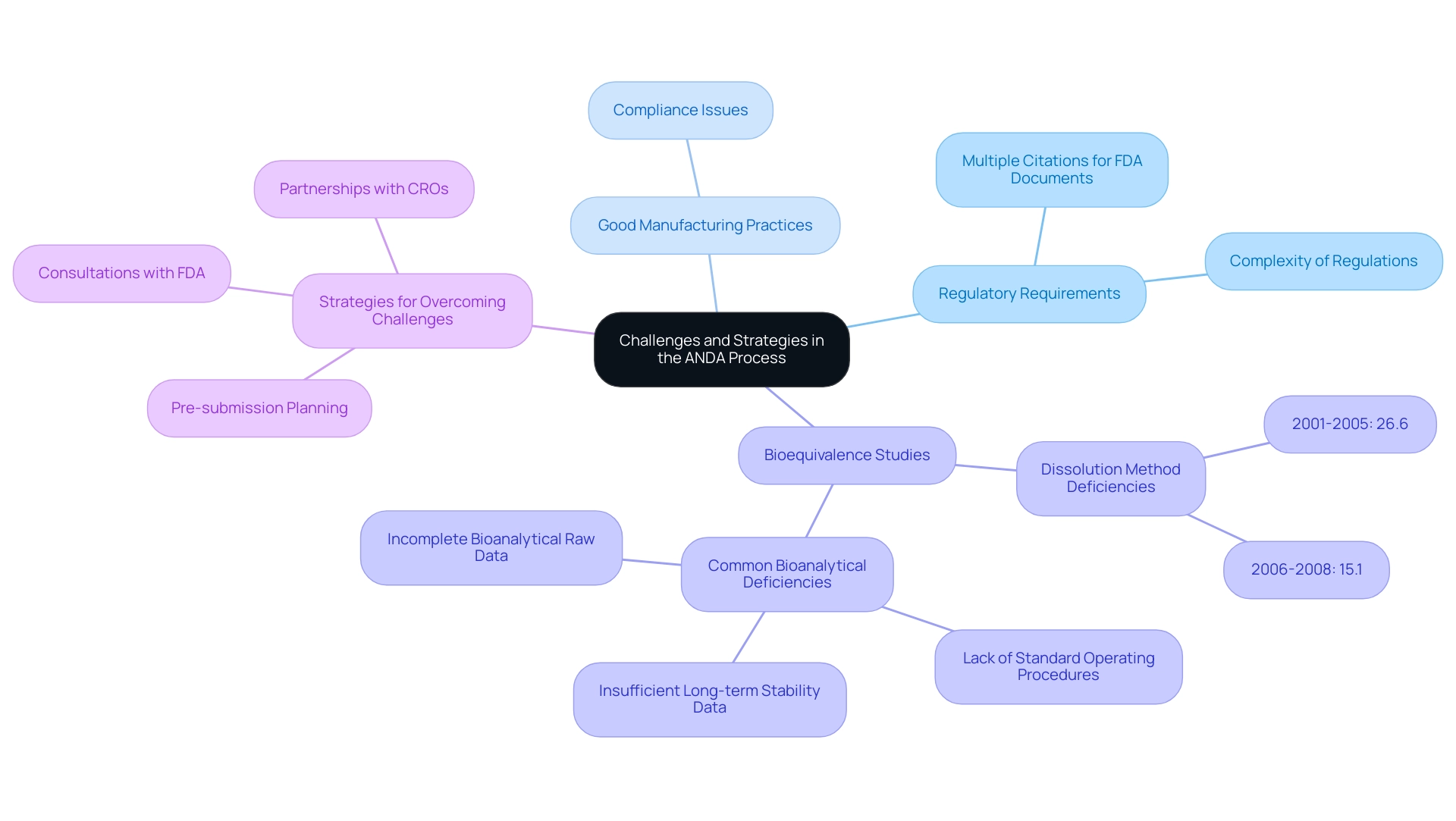
Challenges in the ANDA Process and How to Overcome Them
The abbreviated new drug application process is fraught with challenges, including the navigation of intricate regulatory requirements, compliance with Good Manufacturing Practices, and conducting bioequivalence studies that align with FDA standards. A significant hurdle lies in the necessity for robust data to substantiate bioequivalence, which demands meticulous planning and substantial resources. Notably, statistics indicate that the percentage of applications exhibiting dissolution method deficiencies has significantly decreased from 26.6% during the period of 2001-2005 to 15.1% from 2006-2008, reflecting an ongoing effort to enhance quality of applications.
Furthermore, typical bioanalytical deficiencies observed in drug applications from 2001 to 2008 included:
- A lack of standard operating procedures
- Insufficient long-term stability data
- Incomplete bioanalytical raw data
This highlights the importance of addressing these issues to improve quality. To effectively address these challenges, applicants must engage in comprehensive pre-submission planning, which may include consultations with the FDA and enlisting the expertise of regulatory consultants. As Bryan Coleman, Senior Director for Pharmaceuticals and Medical Devices, advises,
As you prepare your ANDA for filing, understanding what is ANDA in pharmaceuticals may be beneficial, and it might help to enlist an outside regulatory expert to review your packet for any outstanding or conflicting information as well as ensure data accuracy prior to FDA review.
Additionally, nurturing robust partnerships with contract research organizations (CROs) can aid in the execution of bioequivalence studies and accelerate the filing process. The case study on documentation standards emphasizes that adhering to electronic submission guidelines and maintaining well-organized documentation facilitates efficient review by regulatory authorities and reduces misunderstandings. By proactively identifying potential obstacles and implementing strategic planning, pharmaceutical companies can significantly improve their likelihood of securing ANDA approval.
Conclusion
The Abbreviated New Drug Application (ANDA) process plays a critical role in enhancing patient access to affordable medications through the approval of generic drugs. By focusing on demonstrating bioequivalence rather than requiring extensive clinical data, ANDA facilitates a more efficient pathway to market for generic pharmaceuticals. This streamlined approach not only accelerates the availability of lower-cost alternatives but also fosters competition, which in turn contributes to reduced drug prices and improved public health outcomes.
Understanding the intricacies of the ANDA submission process, including the necessity for comprehensive bioequivalence studies and compliance with regulatory standards, is essential for stakeholders in the pharmaceutical industry. The challenges associated with ANDA submissions, such as ensuring robust data and adherence to Good Manufacturing Practices, require meticulous planning and collaboration with regulatory experts. By addressing these challenges through strategic pre-submission planning and fostering partnerships with contract research organizations, applicants can enhance their chances of successful approval.
As the landscape of generic drug approvals evolves with the impending challenges of GDUFA III, it remains imperative for pharmaceutical companies to stay informed and adaptable. The ongoing commitment to transparency and quality in the ANDA process not only strengthens the integrity of generic drug approvals but also ensures that patients continue to benefit from safe, effective, and affordable medication options. The collective impact of these efforts will ultimately shape the future of healthcare accessibility and affordability in the United States.
Frequently Asked Questions
What is an Abbreviated New Drug Application (ANDA)?
An ANDA is the primary route for obtaining FDA authorization for generic medications in the United States. It differs from a New Drug Application (NDA) by streamlining the process to focus on demonstrating bioequivalence to a brand-name drug.
What does bioequivalence mean in the context of ANDAs?
Bioequivalence refers to the requirement that a generic medication delivers the same therapeutic effect as its brand-name counterpart within an equivalent timeframe.
Why are ANDAs important for patients?
ANDAs are crucial for ensuring patient access to affordable medications while maintaining safety and efficacy standards. They also foster competition in the pharmaceutical market, which can lead to lower drug prices.
What are the recent trends in FDA review dynamics for ANDAs?
The FDA has been issuing between 4,150 and 4,500 Information Requests (IRs) annually, with a mix of original applications and supplements. This indicates ongoing challenges and opportunities in the generic drug application process.
What initial steps must applicants take to submit an ANDA?
Applicants must demonstrate that their non-branded medication is pharmaceutically equivalent to the reference listed product (RLD) by providing detailed data on the drug's formulation, manufacturing processes, and labeling.
What is required for the bioequivalence study in an ANDA?
A comprehensive bioequivalence study must show that the generic product has the same rate and extent of absorption as the RLD. Trials with fewer than 600 patients may not allow for subsampling.
What information must be included regarding the manufacturing facility in an ANDA?
The application must confirm that the manufacturing facility adheres to Good Manufacturing Practices (GMP).
What must new owners of an ANDA submit to the FDA?
New owners must either confirm they possess a complete copy of the approved application or request one from the FDA.
What happens after an ANDA is submitted to the FDA?
The FDA conducts a thorough review of the application, which may involve communication with the applicant for clarification or additional information.
What is the significance of successful ANDA approval?
Successful ANDA approval allows for the marketing of alternative medicines, enhancing public health by providing more affordable treatment options.




