Overview
The article primarily aims to serve as a comprehensive guide for mastering the Medtech clinical trial approval process in Ecuador. It underscores the critical need for a thorough understanding of regulatory frameworks, the meticulous preparation of essential documentation, and the unwavering maintenance of compliance throughout both the approval and post-approval stages. This focus is further reinforced by detailed steps and best practices designed to navigate ARCSA's requirements effectively.
Introduction
In the rapidly evolving landscape of medical technology, grasping the regulatory framework in Ecuador is essential for the success of clinical trials. Governed by the ARCSA (Agencia Nacional de Regulación, Control y Vigilancia Sanitaria), the approval process requires a meticulous approach to ensure compliance with updated guidelines that prioritize ethical practices and community engagement.
As new regulations are implemented, stakeholders must adeptly navigate a complex web of documentation, ethical considerations, and compliance requirements, fostering innovation while safeguarding public health.
This article explores the essential steps for preparing, submitting, and conducting clinical trials in Ecuador, offering insights into best practices that can streamline the approval process and enhance the impact of Medtech advancements in the region.
Understand the Regulatory Landscape for Medtech in Ecuador
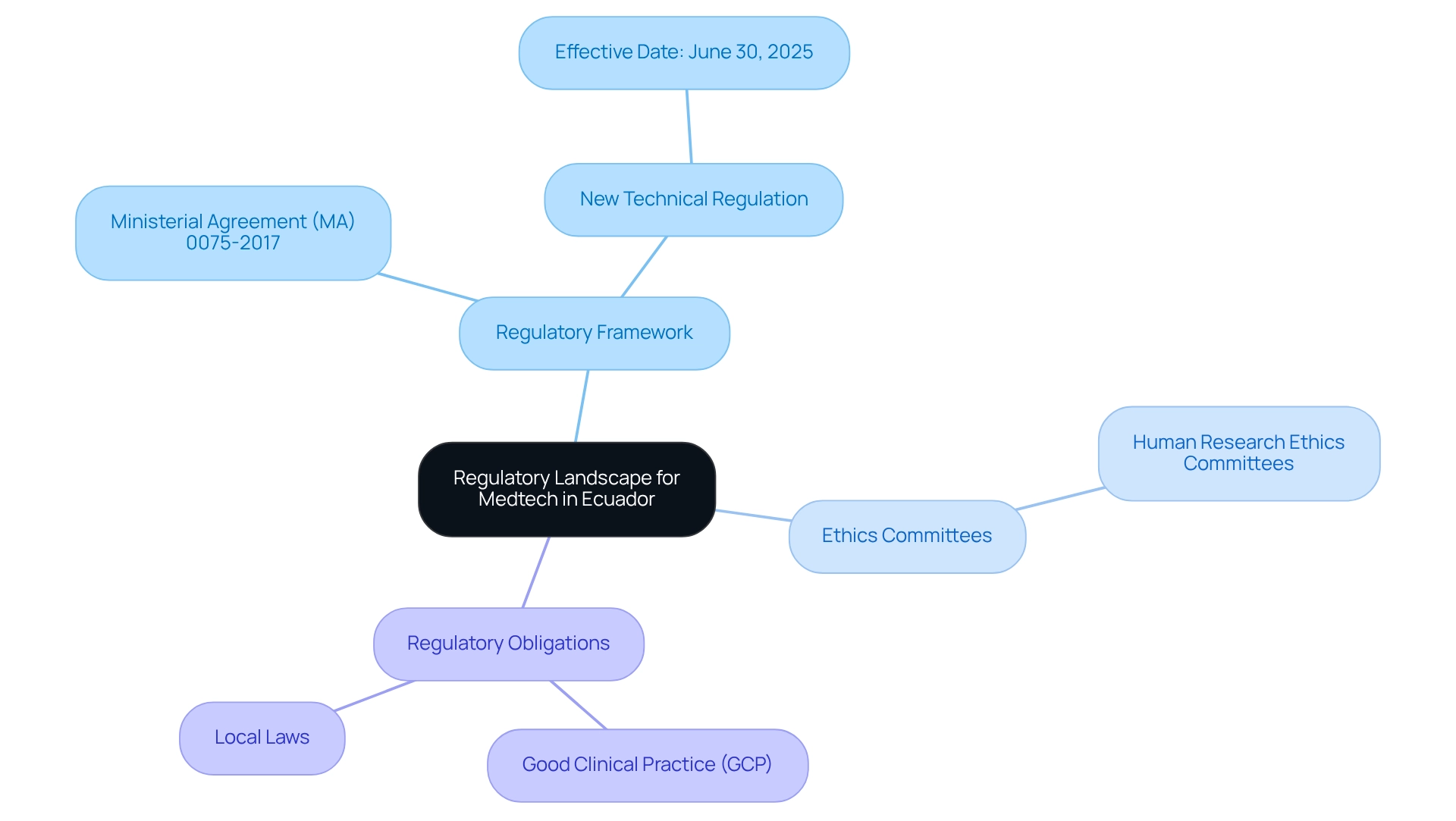
Navigating the Medtech clinical trial approval process in Ecuador necessitates a comprehensive understanding of the regulatory landscape governed by ARCSA (Agencia Nacional de Regulación, Control y Vigilancia Sanitaria). As of 2025, ARCSA has enacted revised guidelines that underscore ethical practices and community involvement, ensuring that research studies are conducted with integrity and transparency. Dr. Patricia Saidón, responsible for research studies at IMT/QR, emphasizes the significance of these regulations for executing thorough and impactful studies that enhance public health.
Key components of this regulatory framework include:
- Regulatory Framework: Familiarize yourself with Ministerial Agreement (MA) 0075-2017 and its amendments, which outline the requirements for conducting clinical trials in Ecuador. Additionally, be aware that a new technical regulation for general medications will take effect on June 30, 2025, potentially influencing the regulatory environment for Medtech studies.
- Ethics Committees: Recognize the essential role of Human Research Ethics Committees in endorsing study protocols, ensuring that ethical standards are upheld throughout the research process.
- Regulatory Obligations: Stay informed about regulatory responsibilities, including adherence to Good Clinical Practice (GCP) guidelines and local laws, which are vital for maintaining the integrity of medical studies.
In this context, bioaccess® is committed to ensuring information security and client trust throughout the research process. With a dedicated Grievance Officer available to address any concerns regarding data protection, bioaccess® emphasizes transparency and compliance with applicable laws. The implementation of new rules, effective December 31, 2024, aims to enhance the rigor and impact of medical studies in Ecuador. These regulations, developed with support from PAHO, establish extensive guidelines for the approval, implementation, and supervision of medical studies, aligning with global ethical standards. The regulation highlights the importance of ethical conduct in research studies, including informed consent procedures and the documentation of serious adverse reactions, as detailed in the case study titled 'Regulation for the Oversight of Research Studies in Ecuador. By comprehending these foundational elements and leveraging the expertise of bioaccess® in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, you will be better positioned to navigate the Medtech clinical trial approval process in Ecuador and contribute to the advancement of Medtech innovations.
Prepare Essential Documentation and Compliance Requirements
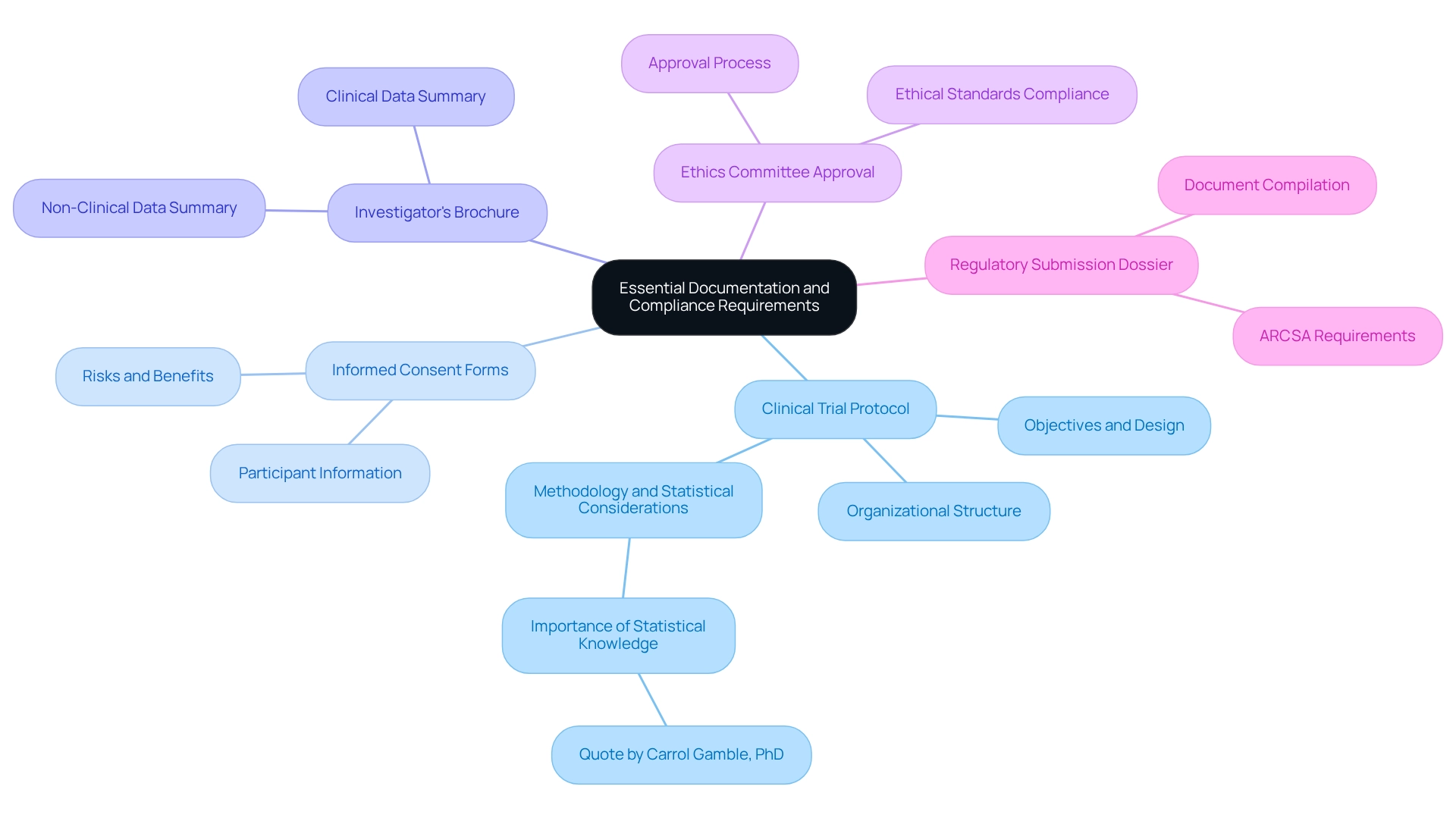
A thorough comprehension of the regulatory environment is vital before commencing the preparation of necessary paperwork for your research application in Latin America. Key components include:
- Clinical Trial Protocol: This detailed plan outlines the trial's objectives, design, methodology, statistical considerations, and organizational structure, serving as the backbone of your study. As Carrol Gamble, PhD, observes, 'The input of the statistician to the design and examination of research studies is recognized as vital,' emphasizing the significance of including statistical knowledge in your protocol.
- Informed Consent Forms: These documents ensure that participants are fully informed about the study's nature, risks, and benefits, and provide their consent to participate.
- Investigator's Brochure: A thorough summary of both clinical and non-clinical data regarding the investigational product, this brochure is vital for informing stakeholders about the product's safety and efficacy.
- Ethics Committee Approval: Obtaining authorization from a recognized ethics committee is a requirement prior to submission, ensuring that the study meets ethical standards.
- Regulatory Submission Dossier: Compile all necessary documents into a submission dossier that adheres to ARCSA's requirements, including any specific forms or templates they mandate.
Navigating the Latin American Medtech landscape requires meticulous preparation and review of these documents to ensure compliance with local regulations, significantly streamlining the approval process. Statistics suggest that non-compliance with documentation standards is a frequent problem in clinical studies, often resulting in delays. For instance, during the COVID-19 pandemic, sponsors created standardized metrics to oversee operational status, including rates of missed visits and protocol deviations. This case study illustrates how effective management of documentation compliance can mitigate challenges. Thus, utilizing professional guidance, such as that from Katherine Ruiz, a specialist in compliance matters for medical devices in Colombia, can offer valuable perspectives on optimal methods for preparing research protocols and documentation in Ecuador. As Scott R. Evans, Ph.D., emphasizes, "Perhaps surprisingly, the most important things that clinicians should know about statistics are not formulas but basic concepts," reinforcing the need for a solid understanding of statistical principles in the preparation of clinical trial documentation.
Submit Applications and Navigate the Approval Process
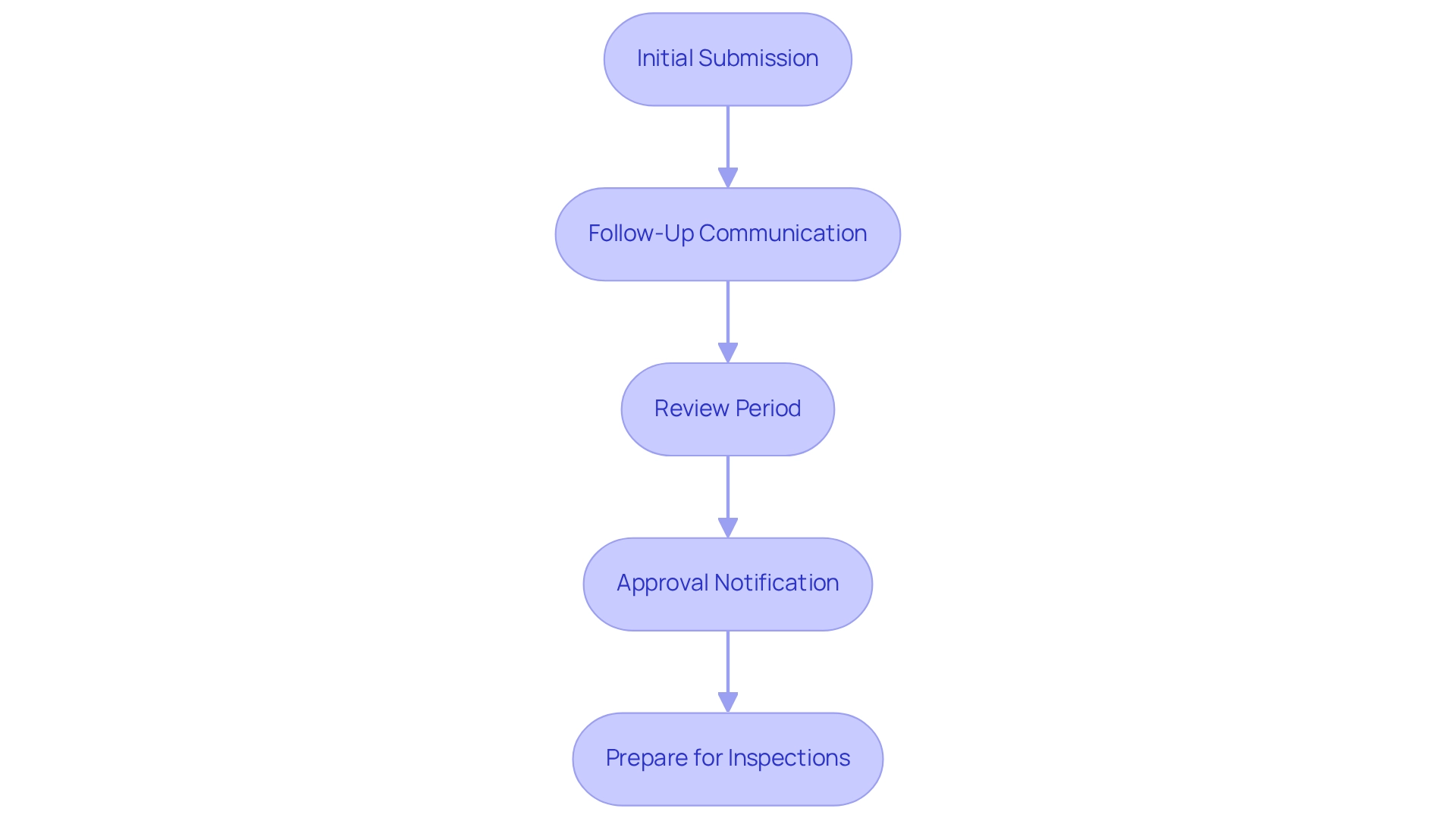
With your documentation prepared, the next step is to submit your application to ARCSA. Follow these steps to navigate the approval process effectively:
- Initial Submission: Begin by submitting your compliance submission dossier through ARCSA's online platform. Ensure that all required documents are included and formatted correctly to avoid delays. ARCSA performs a comprehensive technical review upon application receipt to confirm adherence to Ecuador’s legal specifications.
- Follow-Up Communication: After submission, maintain open lines of communication with ARCSA. Be prepared to respond promptly to any queries or requests for additional information, as this can significantly impact the review timeline.
- Review Period: ARCSA typically takes between 30 to 65 days to review applications. During this period, they may request clarifications or additional data, so it’s crucial to be responsive. After the technical evaluation, ARCSA analyzes medication samples in a laboratory to verify their quality and accuracy in relation to the application data.
- Approval Notification: Upon approval, you will receive a notification from ARCSA. Keep this documentation for your records and future assessments, as it is essential for regulatory adherence.
- Prepare for Inspections: Be ready for potential inspections by ARCSA or ethics committees, which may take place before or during the proceedings. Grasping this facet of the process is crucial for ensuring adherence and preparedness.
As a prominent Contract Research Organization, bioaccess® provides extensive study management services, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting. Our expertise extends to managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). By utilizing our knowledge, you can maneuver through the intricacies of the research approval process in Ecuador more efficiently.
As Daniel P. Logan aptly stated, "Always remember the privilege it is to be a physician." By adhering to these steps, you can maneuver through the approval process more efficiently and reduce possible setbacks, ensuring a more seamless route for your research activities in Ecuador.
Conduct Post-Approval Activities and Ensure Compliance
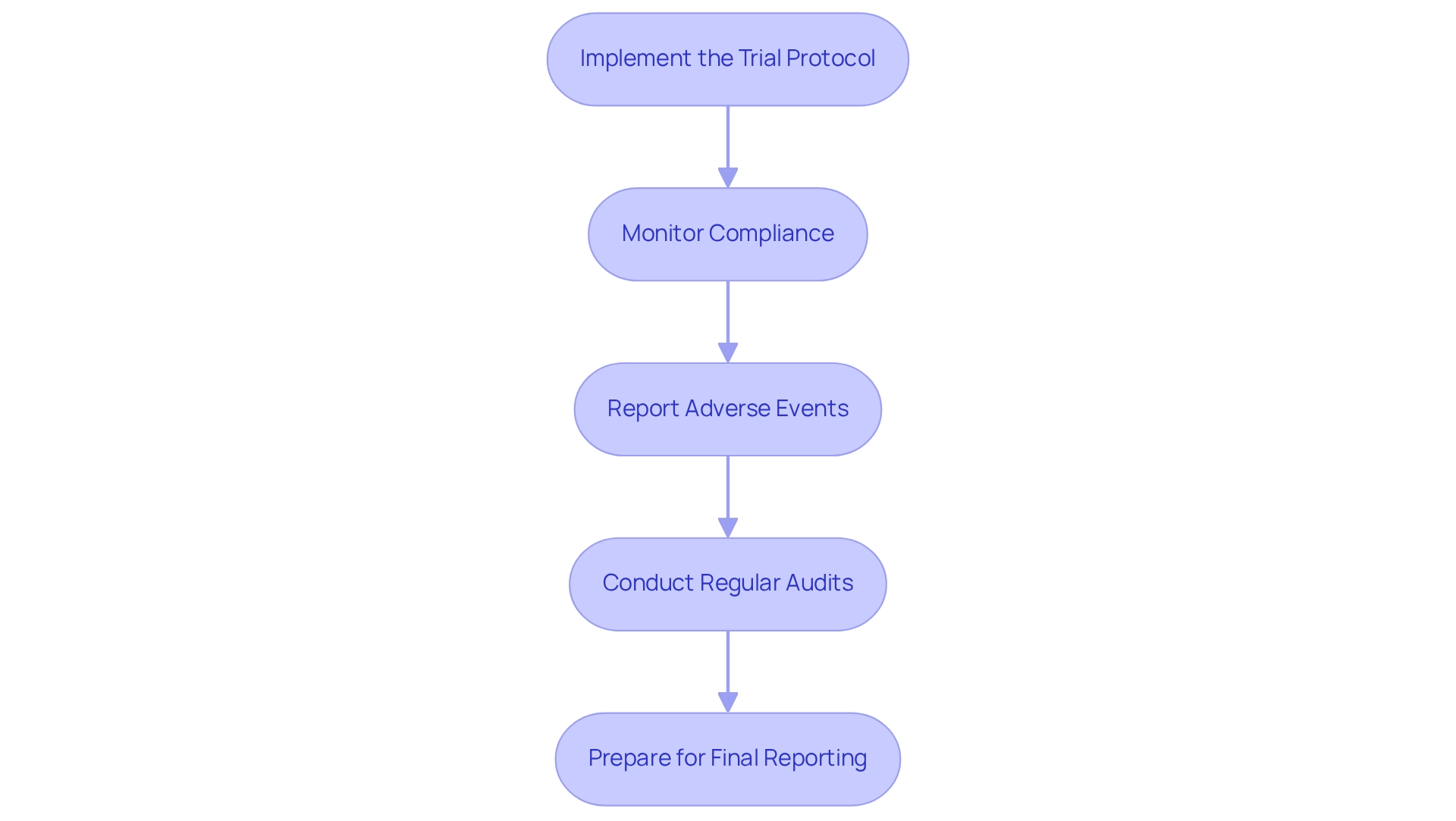
Following the endorsement of your clinical study, executing post-approval tasks is crucial for maintaining compliance with standards set by authorities. Here are the essential steps to follow:
- Implement the Trial Protocol: Initiate the trial in accordance with the approved protocol, ensuring that all team members are adequately trained and fully aware of their responsibilities. Investing in the training and education of personnel involved in the study is essential for upholding compliance with regulations, as it greatly influences the overall success of the study. bioaccess® emphasizes the significance of thorough training to guarantee that all staff are prepared to fulfill the specific compliance standards of the Latin American market.
- Monitor Compliance: Conduct regular reviews of study activities to confirm adherence to the protocol and regulatory requirements. This involves careful oversight of participant safety and data integrity, which is essential for maintaining the credibility of the study. The combination of AI and ML applications has demonstrated a 25% overall enhancement in signal documentation, improving adherence monitoring and data integrity throughout the experiment. bioaccess® employs cutting-edge technologies to simplify regulatory processes and enhance study results.
- Report Adverse Events: Establish a robust system for the prompt reporting of any adverse events or serious adverse events to ARCSA and the ethics committee. Prompt reporting is crucial for ensuring participant safety and meeting regulatory requirements. As Michael Young, Co-Founder, states, "Regulatory adherence requires the use of standardized data gathering techniques and rigorous quality control practices to ensure the accuracy, reliability, and consistency of the data produced during the study." bioaccess® supports clients in developing these systems to ensure swift and accurate reporting.
- Conduct Regular Audits: Schedule internal audits to assess adherence to Good Clinical Practice (GCP) and local regulations. This proactive strategy can assist in recognizing potential problems early, averting escalation and ensuring the integrity of the process. bioaccess® provides extensive audit services to assist clients in upholding high standards of adherence during the testing process.
- Prepare for Final Reporting: At the conclusion of the experiment, compile a comprehensive report summarizing the findings and submit it to ARCSA as required. This concluding report is essential for showcasing adherence and the overall success of the experiment. An evaluation of 158 randomized controlled studies (RCTs) concerning device interventions revealed that merely 13% utilized techniques to address adherence, emphasizing the necessity for heightened awareness about adherence monitoring in research studies.
Diligently conducting these post-approval activities not only ensures the success of your research trial but also reinforces compliance with regulatory standards, ultimately contributing to the advancement of medical technologies through the Medtech clinical trial approval process in Ecuador. With bioaccess® leading the way in clinical research in Latin America, organizations can navigate the complexities of market access with confidence.
Conclusion
Navigating the regulatory landscape for Medtech clinical trials in Ecuador necessitates a robust understanding of ARCSA's guidelines and an unwavering commitment to ethical practices. Familiarity with updated regulations, such as Ministerial Agreement 0075-2017, is essential for ensuring that trials are conducted with integrity and transparency, thereby fostering public trust.
Preparation is paramount; a meticulously structured clinical trial protocol, informed consent forms, and a comprehensive investigator's brochure form the foundation of a strong application. Engaging ethics committees and adhering to Good Clinical Practice (GCP) guidelines further bolster the credibility of the research. Effective documentation management and expert guidance can significantly streamline the approval process and minimize delays.
Post-submission, maintaining open communication with ARCSA and responding promptly to inquiries is vital for expediting the review. Once approval is granted, implementing the trial protocol and ensuring ongoing compliance through regular monitoring and adverse event reporting are crucial for participant safety and trial integrity.
In conclusion, by prioritizing ethical conduct, thorough preparation, and diligent compliance, stakeholders can significantly contribute to the success of clinical trials and advance Medtech innovations in Ecuador. Embracing these best practices not only improves individual trial outcomes but also enhances public health and fosters a culture of innovation in the region.
Frequently Asked Questions
What is the role of ARCSA in the Medtech clinical trial approval process in Ecuador?
ARCSA (Agencia Nacional de Regulación, Control y Vigilancia Sanitaria) governs the regulatory landscape for Medtech clinical trials in Ecuador, ensuring that studies are conducted ethically and transparently.
What are the revised guidelines enacted by ARCSA as of 2025?
The revised guidelines emphasize ethical practices and community involvement in research studies, aiming to enhance public health through integrity and transparency.
What is Ministerial Agreement (MA) 0075-2017?
Ministerial Agreement (MA) 0075-2017 outlines the requirements for conducting clinical trials in Ecuador and includes amendments that need to be understood by those navigating the regulatory framework.
What is the significance of the new technical regulation for general medications?
A new technical regulation for general medications will take effect on June 30, 2025, which may influence the regulatory environment for Medtech studies in Ecuador.
How do Human Research Ethics Committees contribute to clinical trials?
Human Research Ethics Committees endorse study protocols and ensure that ethical standards are upheld throughout the research process.
What are Good Clinical Practice (GCP) guidelines?
Good Clinical Practice (GCP) guidelines are regulatory obligations that must be adhered to in order to maintain the integrity of medical studies.
What is bioaccess®'s commitment to the research process?
Bioaccess® is committed to ensuring information security and client trust throughout the research process, with a dedicated Grievance Officer available to address data protection concerns.
What new rules are set to be implemented on December 31, 2024?
New rules aimed at enhancing the rigor and impact of medical studies in Ecuador will be implemented, developed with support from PAHO, and will establish extensive guidelines for approval, implementation, and supervision of medical studies.
What are the key aspects of ethical conduct in research studies?
Key aspects include informed consent procedures and the documentation of serious adverse reactions, which are essential for maintaining ethical standards in research.
How can expertise from bioaccess® assist in navigating the Medtech clinical trial approval process?
Leveraging bioaccess®'s expertise in managing various types of studies, such as Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, can help navigate the approval process and advance Medtech innovations.




