Overview
The article addresses the critical aspects of navigating the Medtech trial approval process in Paraguay, underscoring the necessity of comprehending regulatory requirements and ethical considerations. It outlines essential steps, including:
- Preparation of documentation
- Engagement with ethics committees
- Utilization of local networks
Furthermore, it brings to light the challenges presented by corruption and emphasizes the imperative of compliance throughout the trial, reinforcing the relevance of these factors in clinical research.
Introduction
In the rapidly evolving field of medical technology, understanding the regulatory landscape is crucial for successfully conducting clinical trials, particularly in emerging markets like Paraguay. As the demand for innovative healthcare solutions grows, the complexity of navigating the approval processes set forth by the Ministry of Health also increases.
From the establishment of a comprehensive regulatory framework to the imperative role of ethics committees, this landscape presents both challenges and opportunities. With anticipated changes on the horizon and a burgeoning medical device market, stakeholders must adopt strategic approaches to ensure compliance and streamline trial operations.
This article delves into the essential components of the Medtech regulatory environment in Paraguay, offering insights into effective strategies for trial approval, participant recruitment, and data management, all aimed at fostering successful outcomes in this promising region.
Understand the Medtech Regulatory Landscape in Paraguay
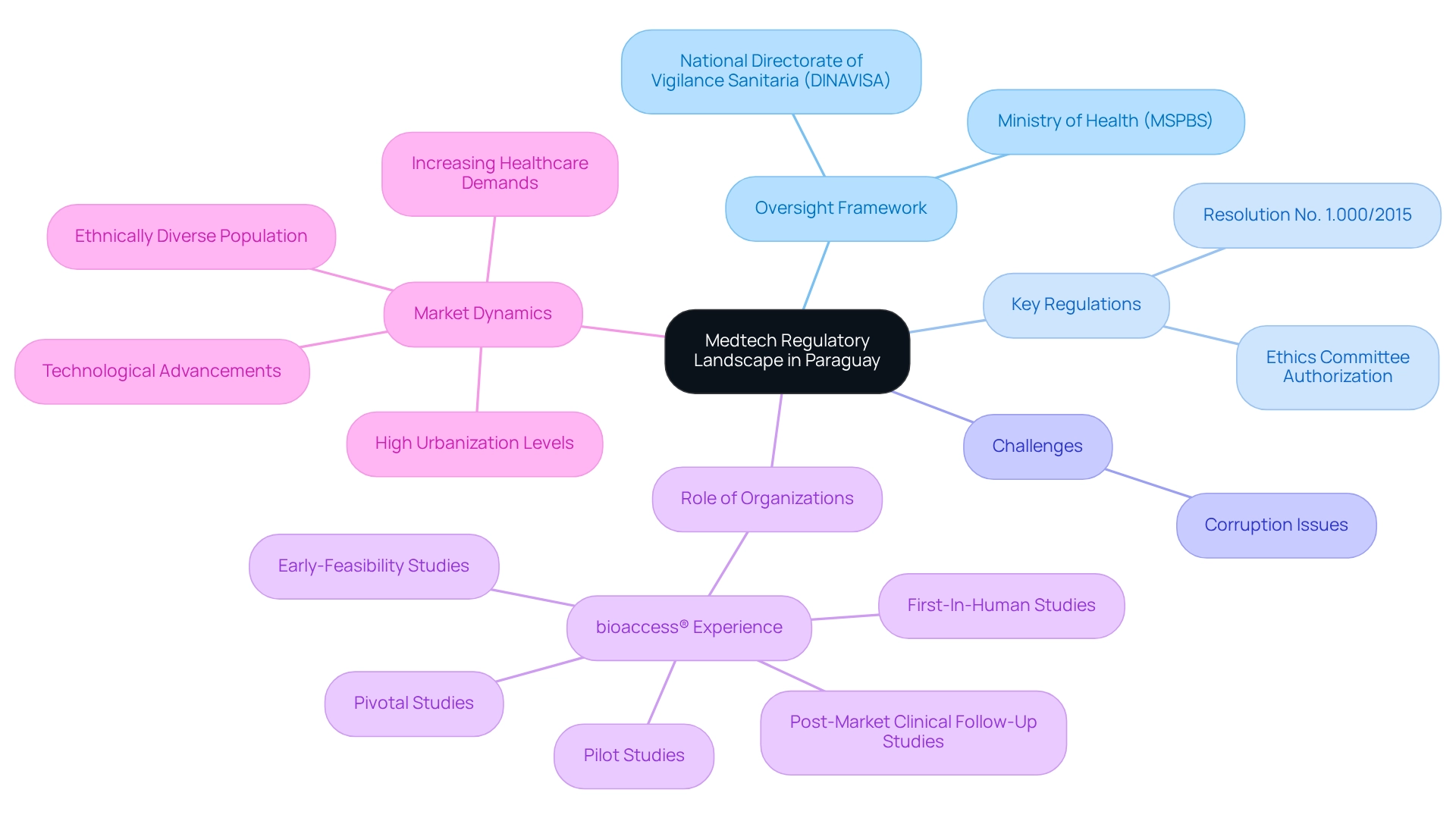
Navigating the Medtech trial approval process in Paraguay requires a comprehensive understanding of the oversight framework, primarily governed by the Ministry of Health (MSPBS). The oversight structure in Paraguay is robust, encompassing laws and resolutions that guide the approval process for medical devices. Significant regulatory changes are expected in 2024, which may further refine these processes.
Key regulations such as Resolution No. 1.000/2015 delineate the requirements for medical studies, complemented by guidelines from the National Directorate of Vigilance Sanitaria (DINAVISA). Authorization from an ethics committee is crucial for all medical studies, ensuring the protection of participants' rights and welfare. However, it is essential to acknowledge that corruption remains a significant challenge in Paraguay's clinical study oversight process, posing obstacles for researchers and sponsors. Comprehensive documentation is vital, including study protocols, informed consent forms, and safety data, to demonstrate compliance with local regulations.
Understanding these elements is imperative for effectively navigating the approval process. The Paraguayan government is increasingly recognizing the importance of collaboration and alignment in biotechnology oversight to enhance the overall governance framework for the Medtech trial approval process in Paraguay. Furthermore, with bioaccess®'s over 20 years of experience in managing Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies, navigating these regulatory requirements becomes more streamlined. Their tailored approach further assists clients in overcoming challenges. The ethnically diverse population and high urbanization levels in Latin America position the region as an attractive site for initial feasibility studies, underscoring the potential for successful research approvals in Paraguay. Notably, the medical device market in the country is thriving, driven by increasing healthcare demands and rapid technological advancements, making it a compelling area for conducting clinical studies.
Navigate Regulatory Requirements for Medtech Trials
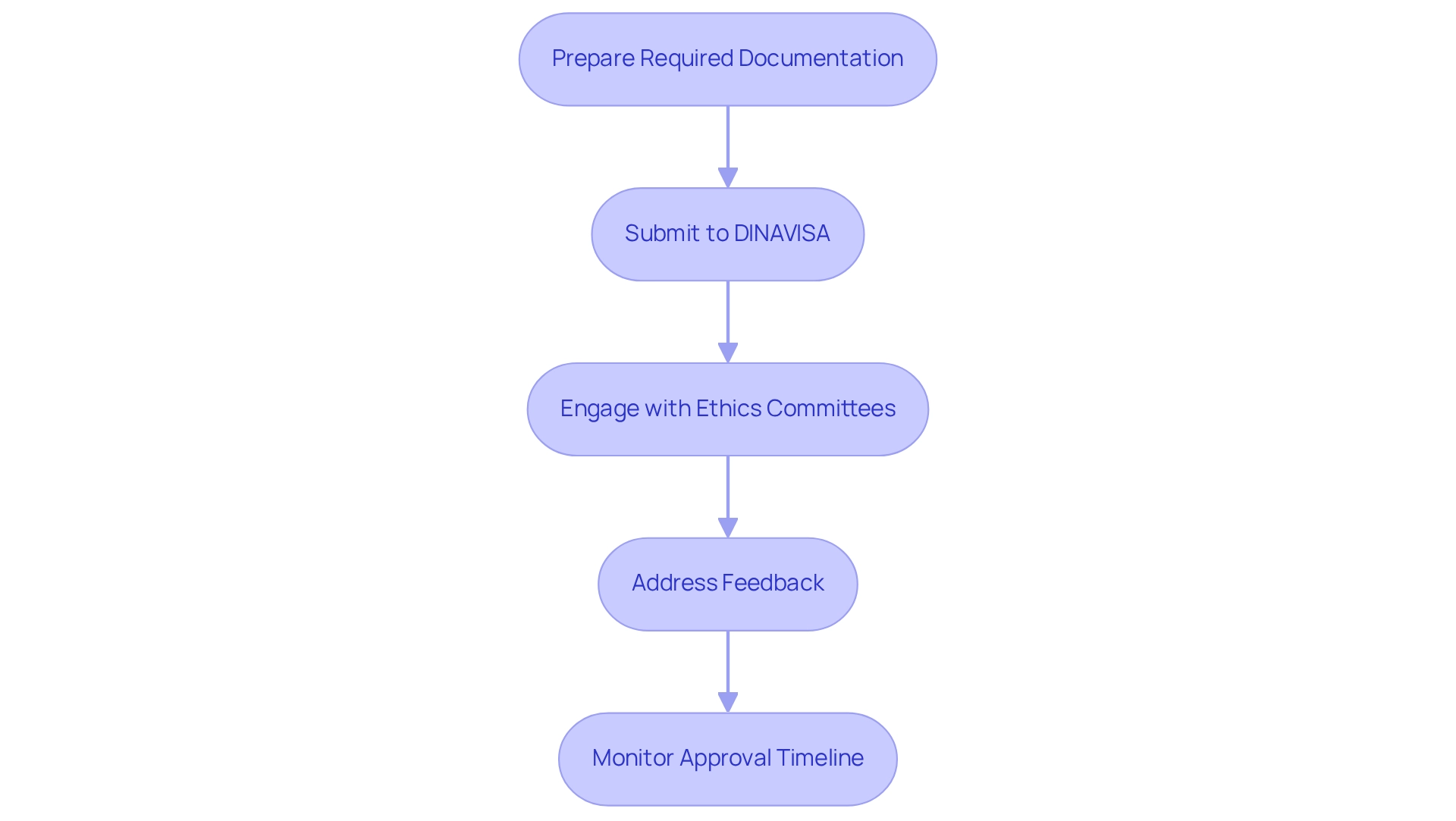
Navigating the regulatory requirements for Medtech studies in Paraguay necessitates a systematic approach to ensure compliance and expedite the Medtech trial approval process in Paraguay. The essential steps to follow are as follows:
-
Prepare Required Documentation: Compile all necessary documents, including the clinical trial protocol, investigator's brochure, informed consent forms, and safety and efficacy data.
-
Submit to DINAVISA: Submit your application to DINAVISA, ensuring that all required documentation is complete to prevent delays in processing.
-
Engage with Ethics Committees: Secure approval from an ethics committee by submitting your study protocol and informed consent forms for their review.
-
Address Feedback: Be prepared to respond to any feedback or requests for additional information from DINAVISA or the ethics committee. This may involve revising your documentation or providing further data to meet their requirements.
-
Monitor Approval Timeline: Keep a close watch on the approval timeline and maintain open communication with oversight organizations to facilitate a smooth process.
Incorporating perspectives from the case study titled 'Regulatory Affairs: CRO-Sponsor Interaction,' early involvement between CROs such as bioaccess and sponsors is crucial for compliance success. This collaboration fosters a strategic alliance that enhances the effectiveness of medical studies, including services such as feasibility assessments, selection of research locations and lead investigators, study set-up, and project management. By diligently following these steps and leveraging collaboration with regulatory authorities, you can effectively navigate the regulatory landscape and expedite the Medtech trial approval process in Paraguay. Successful submissions often hinge on early involvement, promoting a cooperative atmosphere that enhances the effectiveness of research studies.
Recruit Participants for Clinical Trials Effectively
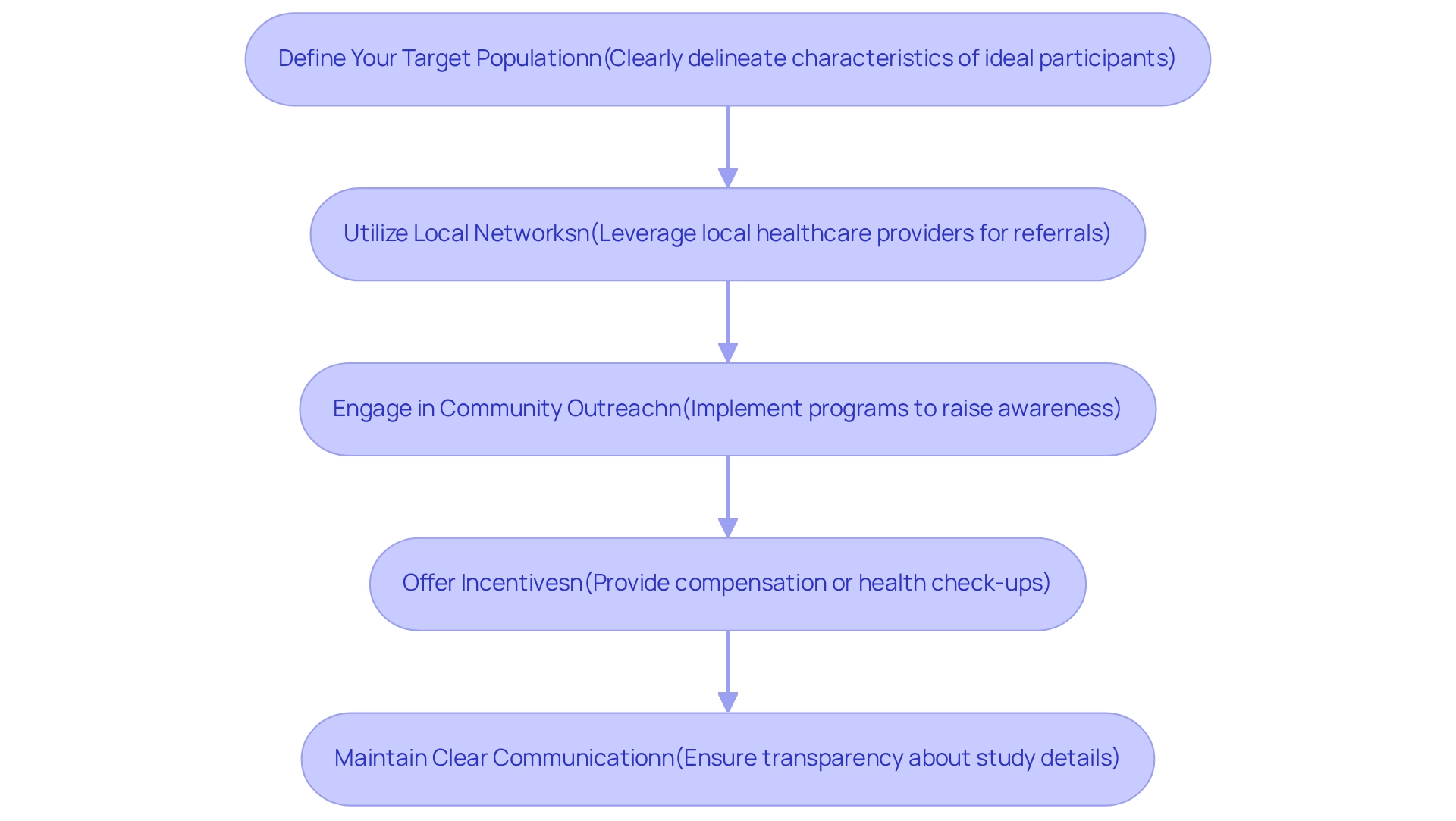
Enlisting individuals for research studies in Paraguay necessitates a strategic and well-organized method as part of the Medtech trial approval process in Paraguay. To enhance your recruitment efforts effectively, consider the following strategies:
-
Define Your Target Population: Clearly delineate the characteristics of your ideal participants based on the study's objectives, including age, gender, health status, and other pertinent factors. This targeted approach ensures that recruitment efforts remain focused and efficient.
-
Utilize Local Networks: Leverage the existing infrastructure of local healthcare providers, clinics, and hospitals to identify and reach potential participants. Establishing partnerships with these entities can facilitate referrals and enhance recruitment efforts. For instance, GlobalCare Clinical Studies has successfully collaborated with bioaccess™ to improve its clinical study ambulatory services in Colombia, resulting in a decrease in recruitment time by over 50% and a retention rate exceeding 95%. This underscores how local collaborations can significantly enhance recruitment outcomes.
-
Engage in Community Outreach: Implement outreach programs within communities to raise awareness about the examination. Utilize informational sessions, flyers, and social media campaigns to inform potential participants about the benefits and significance of the study, fostering a sense of community involvement. Collaborating with patient advocacy groups can enhance trust and engagement in the recruitment process, leading to better outreach and alignment with patient needs. Effective community outreach can mirror the successful strategies employed by GlobalCare Clinical Trials in Colombia, where local engagement played a crucial role in participant recruitment.
-
Offer Incentives: Consider providing incentives for participation, such as compensation for travel expenses or complimentary health check-ups. These incentives can significantly boost enrollment rates by making participation more appealing.
-
Maintain Clear Communication: Ensure that all communications are transparent and straightforward. Providing potential participants with thorough details about the study, including risks and benefits, is essential for fostering trust and promoting involvement. As Ursula Türcke, Senior Director of Clinical Operations at FGK Clinical Research GmbH, noted, AstraZeneca's 'no cure – no pay' approach emphasizes the importance of clear communication in fostering trust with participants. This principle is vital in the context of recruitment approaches, as demonstrated by the successful results of GlobalCare Clinical Studies, which relate to the Medtech trial approval process in Paraguay. By applying these approaches, you can efficiently enlist participants and significantly improve the overall success of your research in Paraguay. With approximately 2,092 active studies currently in Phase III, the demand for effective recruitment strategies is more critical than ever, particularly given the country's favorable demographics and healthcare access. Furthermore, as emphasized by Clinical Leader, the expanding media attention on medical studies in Latin America highlights the rising significance of these investigations in promoting economic development and healthcare advancements in the area.
Ensure Data Management and Compliance Throughout the Trial
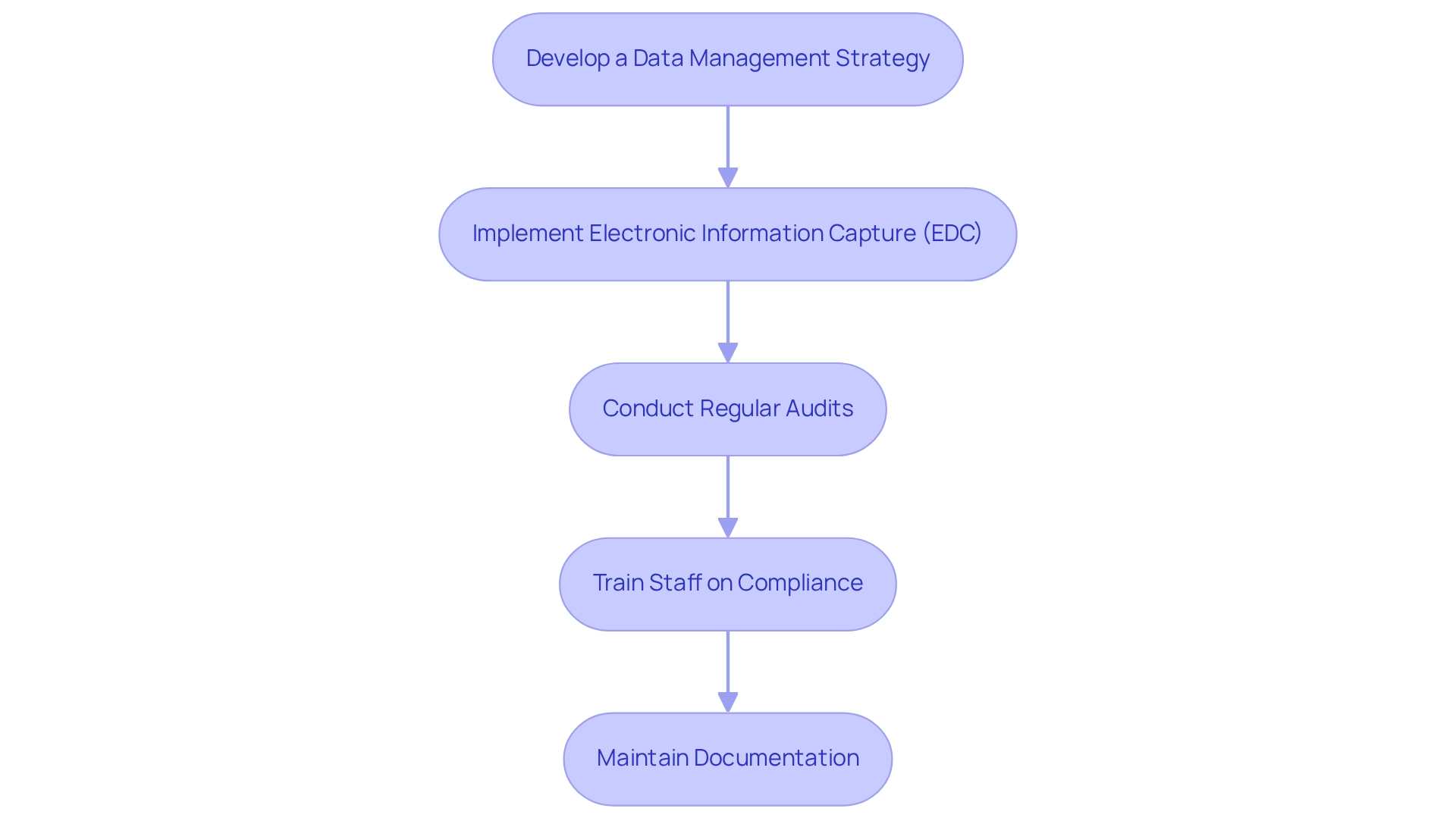
Efficient information management and adherence are crucial throughout the clinical study process. Adhering to regulatory standards not only enhances the credibility of your trial but also ensures successful outcomes. Here are key guidelines to follow:
-
Develop a Data Management Strategy: Formulate a comprehensive management plan detailing how information will be collected, stored, and analyzed. This plan should encompass data collection methods, data storage solutions, and data security measures.
-
Implement Electronic Information Capture (EDC): Utilize EDC systems to simplify information collection and minimize errors. These systems not only improve efficiency but also guarantee adherence to legal requirements concerning integrity and security. Organizations that implement comprehensive information management solutions can shorten their drug development cycle by an average of 1.5 years and enhance their chances of receiving approval by 23%. Furthermore, EDC systems securely store information, ensuring compliance with privacy laws such as HIPAA and GDPR.
-
Conduct Regular Audits: Schedule routine evaluations of information management practices to verify compliance with regulatory standards and internal protocols. This proactive approach helps identify and address any issues promptly, ensuring the integrity of the trial. The urgency and accountability shown by the Data Monitoring Committee (DMC) in the RECOVERY Trial illustrate the essential nature of prompt decision-making and comprehensive analysis in upholding compliance and information integrity.
-
Train Staff on Compliance: Provide comprehensive training for all team members involved in information management. Ensuring that staff understand compliance requirements and best practices is crucial for maintaining high standards throughout the trial.
-
Maintain Documentation: Keep meticulous records of all information management processes, including entry logs, audit trails, and compliance reports. This documentation is essential for regulatory inspections and audits, reinforcing the study's credibility. The DMC's charter, which directs decision-making processes concerning treatment effectiveness and participant safety, emphasizes the significance of structured governance in information management and compliance.
In addition to these practices, bioaccess provides comprehensive research management services, including feasibility studies, site selection, compliance evaluations, setup, import permits, project supervision, and reporting. By prioritizing these data management and compliance practices, along with leveraging bioaccess's expertise, you can significantly enhance the integrity of your clinical trial and contribute to its overall success.
Conclusion
Understanding the regulatory landscape for Medtech trials in Paraguay is essential for success in this dynamic environment. Key components, such as a robust regulatory framework, the necessity of ethics committee approval, and comprehensive documentation requirements, play a vital role in navigating the approval process. Stakeholders must remain vigilant about potential corruption issues while also adapting to the anticipated regulatory changes in 2024 that could further streamline these processes.
Effective participant recruitment strategies are crucial, as they directly impact the success of clinical trials. By defining target populations, leveraging local networks, engaging in community outreach, and maintaining clear communication, researchers can significantly enhance their recruitment efforts. The demonstrated benefits of collaboration with local healthcare providers illustrate the importance of community involvement in achieving successful outcomes.
Moreover, ensuring data management and compliance throughout the trial is paramount. Developing a solid data management plan, implementing electronic data capture systems, and conducting regular audits help maintain the credibility and integrity of the trial. Coupled with thorough staff training and meticulous documentation, these practices fortify the overall success of clinical trials in Paraguay.
In conclusion, as the demand for innovative healthcare solutions continues to rise, stakeholders in Paraguay's Medtech sector must embrace these strategies to navigate the complexities of clinical trials. By prioritizing regulatory compliance, effective recruitment, and robust data management, the potential for successful outcomes in this burgeoning market becomes increasingly attainable.
Frequently Asked Questions
What is the primary governing body for Medtech trial approval in Paraguay?
The primary governing body for Medtech trial approval in Paraguay is the Ministry of Health (MSPBS).
What are some key regulations that guide the approval process for medical devices in Paraguay?
Key regulations include Resolution No. 1.000/2015, which outlines the requirements for medical studies, along with guidelines from the National Directorate of Vigilance Sanitaria (DINAVISA).
Why is authorization from an ethics committee important in the approval process?
Authorization from an ethics committee is crucial for all medical studies to ensure the protection of participants' rights and welfare.
What challenges does the clinical study oversight process in Paraguay face?
A significant challenge in the clinical study oversight process in Paraguay is corruption, which poses obstacles for researchers and sponsors.
What documentation is essential for compliance with local regulations?
Essential documentation includes study protocols, informed consent forms, and safety data.
How is the Paraguayan government addressing the Medtech trial approval process?
The Paraguayan government is recognizing the importance of collaboration and alignment in biotechnology oversight to enhance the overall governance framework for the Medtech trial approval process.
How does bioaccess® assist in navigating regulatory requirements?
Bioaccess® has over 20 years of experience in managing various types of studies, which helps streamline the navigation of regulatory requirements through a tailored approach.
What makes Paraguay an attractive site for initial feasibility studies?
Paraguay is considered an attractive site for initial feasibility studies due to its ethnically diverse population and high urbanization levels in Latin America.
What factors are contributing to the growth of the medical device market in Paraguay?
The medical device market in Paraguay is thriving due to increasing healthcare demands and rapid technological advancements.




