Overview
The article delves into the intricate regulatory pathways for the approval of healthcare instruments in Argentina, primarily governed by the National Administration of Drugs, Food and Medical Devices (ANMAT). Understanding device classification, compliance requirements, and the dynamic regulatory landscape is essential.
Thorough preparation and adherence to established guidelines are paramount for achieving successful market entry and fostering innovation within the healthcare technology sector.
Introduction
In the intricate landscape of medical device regulation in Argentina, grasping the nuances of the approval process is essential for success. The National Administration of Drugs, Food and Medical Devices (ANMAT) is continually refining its regulatory framework, presenting both challenges and opportunities for manufacturers aiming to bring innovative solutions to market.
Classifying devices based on risk and compiling comprehensive documentation are critical steps in the regulatory submission process, each playing a vital role in ensuring compliance and efficiency. As the medical technology market thrives, organizations must arm themselves with the knowledge and resources necessary to navigate this evolving terrain effectively.
This article explores the essential components of the regulatory framework, preparation strategies, documentation requirements, and post-approval compliance measures, providing a roadmap for medical device manufacturers seeking to excel in Argentina's dynamic healthcare sector.
Understand the Regulatory Framework in Argentina
Navigating the regulatory pathways for approval in Argentina for healthcare instruments necessitates a thorough understanding of the compliance framework's key elements. The National Administration of Drugs, Food and Medical Devices (ANMAT) is the principal governing body overseeing these regulatory pathways. Familiarity with the regulatory pathways for approval is essential, as ANMAT evaluates and approves health-related products. The legislation delineates these pathways, with significant laws such as Decree 1490/92 outlining compliance requirements for medical equipment and establishing a legal framework for adherence. Medical devices are classified into four categories (I, II, III, IV) based on their risk levels, directly influencing the regulatory pathways for approval and the corresponding requirements. Staying informed about ANMAT's guidelines is crucial, as any changes can significantly impact the submission process.
As we look to 2025, the compliance landscape is evolving, with ANMAT streamlining regulatory pathways to enhance efficiency. Recent statistics indicate that successful navigation of these pathways can result in expedited approvals, underscoring a growing commitment to fostering innovation within the healthcare technology sector. The global medical technology market is valued at over US$567 billion annually, highlighting the importance of effectively maneuvering through the compliance framework.
Entities like bioaccess® exemplify how leading contract research organizations in Latin America assist Medtech startups in understanding the regulatory pathways for approval in Argentina, facilitating the swift progression of medical devices from early-feasibility studies to commercialization. With over 20 years of expertise, bioaccess® oversees a variety of studies, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
Ensuring adherence and efficiency throughout the clinical trial process. Furthermore, tools like RegDesk have revolutionized application preparation, reducing the duration from six months to just six days, significantly aiding compliance initiatives.
Understanding these components will enable you to effectively prepare for the submission process and ensure adherence to the regulatory pathways for approval in Argentina.
Prepare for the Regulatory Submission Process
Preparation for the compliance submission process involves several critical steps:
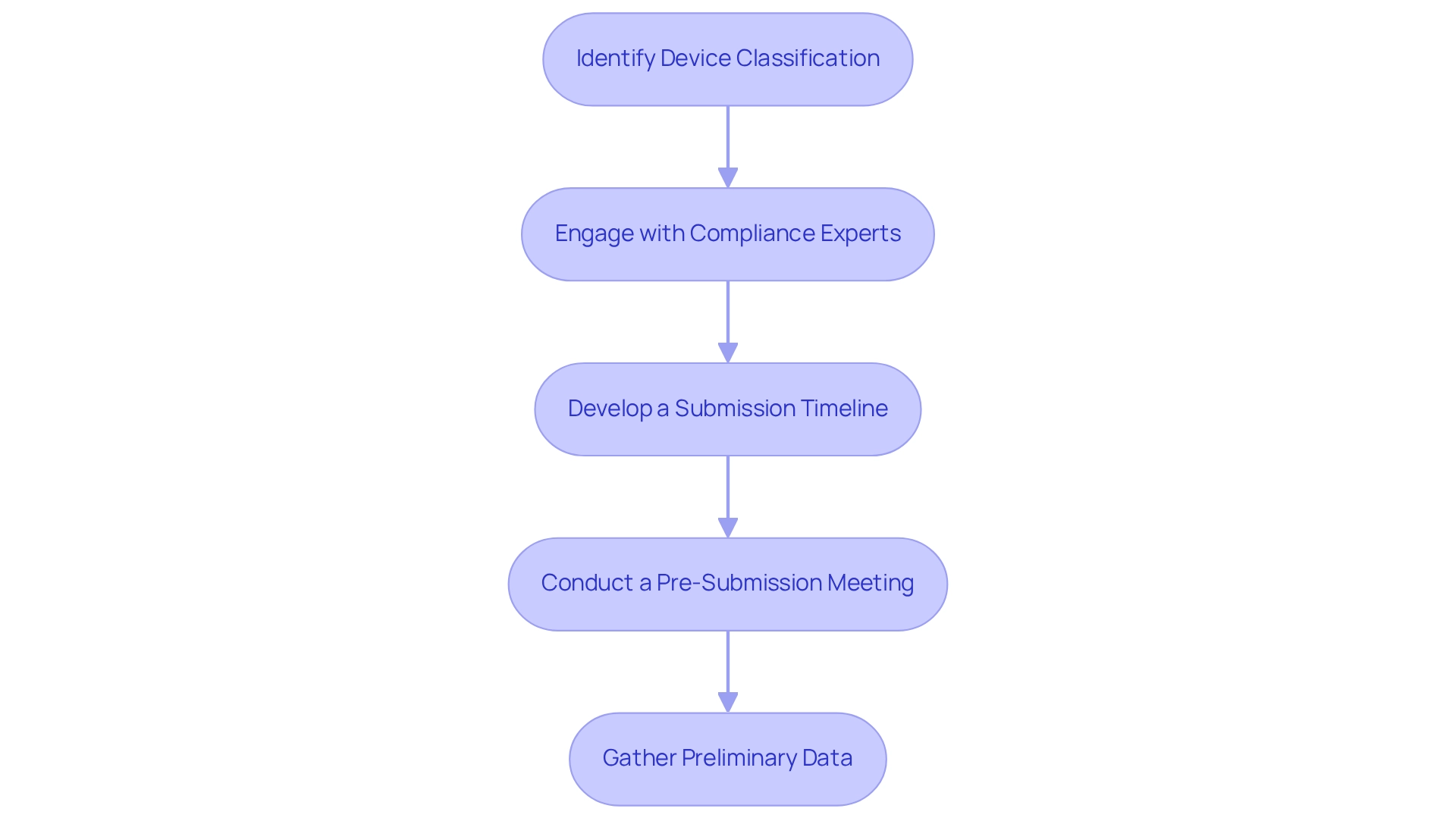
- Identify the Device Classification: Determine whether your device falls under Class I, II, III, or IV. This classification outlines the specific requirements and timelines for approval, which are essential for successfully navigating the regulatory pathways for approval in Argentina.
- Engage with Compliance Experts: Consulting with affairs specialists experienced with ANMAT can provide invaluable guidance throughout the submission process. Their expertise can help streamline your approach and ensure adherence to local regulations.
- Develop a Submission Timeline: Create a detailed timeline outlining all necessary steps leading up to the submission, including document preparation, internal reviews, and any required consultations. A well-organized timeline is crucial for meeting compliance deadlines and preventing delays.
- Conduct a Pre-Submission Meeting: Arrange a meeting with ANMAT to discuss your submission plan. This proactive step can clarify uncertainties and align expectations, ultimately enhancing the likelihood of a smooth approval process.
- Gather Preliminary Data: Collect preliminary data or studies that support your submission, including clinical data, safety assessments, and efficacy studies. This information is essential for showcasing the equipment's worth and adherence to compliance standards.
The healthcare market in Argentina is shaped by government policies that promote innovation and investment in the health sector, which include regulatory pathways for approval in Argentina, generating substantial opportunities for producers. As emphasized by new patterns in clinical trials, there is a transition toward adaptive frameworks and the incorporation of digital health technologies, which may affect compliance submissions.
Moreover, the COVID-19 pandemic has greatly influenced healthcare expenditure in Argentina, resulting in heightened demand for essential healthcare tools. This context highlights the significance of prompt compliance submissions to take advantage of market opportunities.
By adhering to these steps, you will create a strong basis for your compliance submission, reducing possible obstacles and positioning your medical device for successful market entry by navigating the regulatory pathways for approval in Argentina. bioaccess® provides extensive clinical trial management services, such as feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting, ensuring that you are well-prepared for the challenges ahead and can maneuver through the compliance landscape effectively.
Compile Required Documentation and Ensure Compliance
Assembling the necessary documentation is essential for a successful compliance submission. Ensure that your submission includes the following key components:
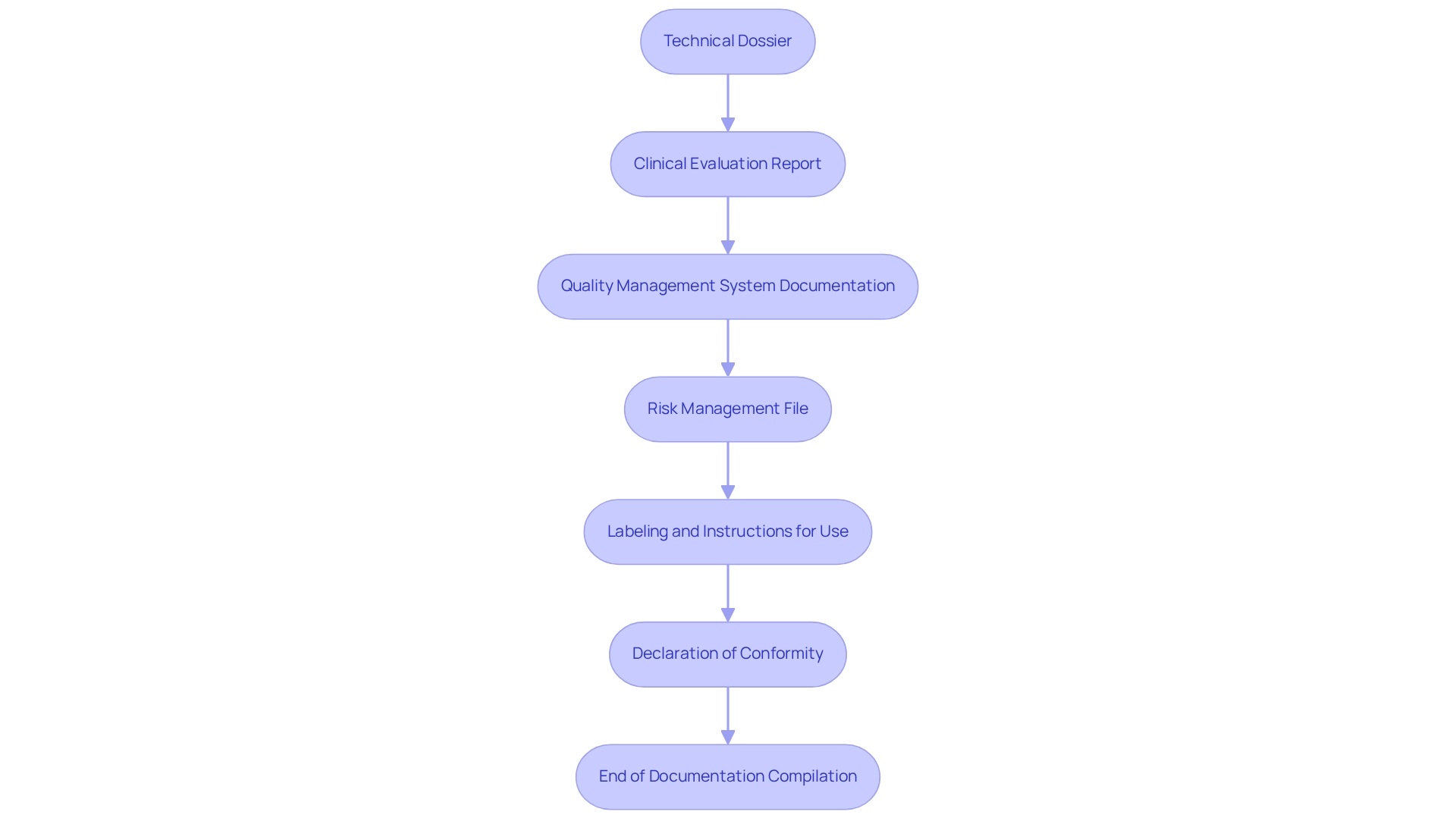
- Technical Dossier: Create a comprehensive technical dossier that includes detailed information about the apparatus, its intended use, and the manufacturing processes involved. This dossier is essential for demonstrating compliance with legal standards.
- Clinical Evaluation Report: Include a comprehensive report that summarizes clinical data, showcasing the safety and efficacy of the product. This report is essential, as successful clinical evaluation reports are a cornerstone of the regulatory pathways for approval in Argentina.
- Quality Management System Documentation: Provide evidence of adherence to quality management standards, such as ISO 13485. This documentation reassures regulators of your commitment to quality and safety. As highlighted in a case study on ANMAT Good Manufacturing Practices, understanding the regulatory pathways for approval in Argentina is crucial for aligning Argentinian quality system requirements with US FDA regulations to ensure successful audits.
- Risk Management File: Document the risk evaluation and management strategies applied throughout the product's development. This file should reflect a proactive approach to identifying and mitigating potential risks associated with the device.
- Labeling and Instructions for Use: Ensure that all labeling meets ANMAT regulations, including clear instructions for use and safety information. Proper labeling is essential for user safety and adherence to regulations.
- Declaration of Conformity: Submit a declaration affirming that the equipment meets all relevant regulatory requirements. This declaration is a formal acknowledgment of your equipment's adherence to the necessary standards.
By meticulously compiling these documents, you significantly enhance the likelihood of a smooth review process. Significantly, the formal evaluation period for all categories of health equipment is established at 180 days; however, real evaluation durations can stretch to approximately one year. For Class I and II items, registration review takes approximately 15 to 30 working days. Therefore, thorough preparation is essential to navigate this timeline effectively. Furthermore, grasping the financial consequences of adhering to regulations is crucial, as imported healthcare instruments in Argentina are subject to the Mercosur Common External Tariff, which specifies tax rates between 0% and 16%, along with a value-added tax (VAT) of 10.5% for new items and 21% for pre-owned or refurbished items. Utilizing the knowledge of bioaccess® in overseeing clinical trials can further simplify this process, guaranteeing adherence and successful results in the legal environment of Latin America.
Implement Post-Approval Monitoring and Compliance Strategies
Implementing effective post-approval monitoring and compliance strategies is essential once your medical device has received regulatory approval.
- Establish a Post-Market Surveillance Plan: Create a comprehensive plan to monitor your product's performance in the market, which includes tracking adverse events and gathering user feedback. This proactive approach is vital for maintaining device safety and effectiveness. Regular updates to this plan should reflect new safety concerns and ensure appropriate actions are taken.
- Regular Reporting to INVIMA: Comply with the requirement of promptly reporting any adverse events or safety issues to INVIMA. The emphasis on accurate and prompt reporting is critical, as statistics indicate that healthcare organizations may spend upwards of $500,000 monthly on additional resources to manage increasing transaction volumes.
- Conduct Periodic Audits: Implement a schedule for regular audits of your quality management system to ensure compliance with regulatory requirements set forth by INVIMA. These audits help identify and address potential issues before they escalate, reinforcing your commitment to safety.
- Engage with Healthcare Professionals: Foster open communication with healthcare providers using your technology. Their insights can be invaluable in identifying concerns and enhancing performance in practical environments. bioaccess® can assist in this engagement by providing tailored clinical research services that facilitate effective communication and feedback collection.
- Update Documentation as Necessary: Ensure that all documentation, including labeling and instructions for use, is current and reflects any changes based on post-market data. This practice not only aids compliance but also enhances user understanding and safety.
By adopting these strategies, you will not only meet regulatory requirements but also significantly enhance the safety and effectiveness of your medical product in the market. As emphasized by industry experts, "By being proactive and constantly mitigating risk, you can ultimately keep your device on the market longer." The integration of post-market surveillance activities into your overall quality management system is crucial for ongoing compliance and risk mitigation.
Conclusion
Successfully navigating the regulatory landscape for medical devices in Argentina necessitates a profound understanding of the processes governed by the National Administration of Drugs, Food and Medical Devices (ANMAT). Manufacturers must thoroughly familiarize themselves with regulatory bodies, legislation, and guidelines to ensure compliance and facilitate a smooth submission process.
Preparation is paramount, involving the establishment of a clear submission timeline, consultation with regulatory experts, and the compilation of critical documentation such as technical dossiers and clinical evaluation reports. These essential steps position organizations for successful market entry while leveraging opportunities within the healthcare sector.
Post-approval, it is crucial to implement effective monitoring and compliance strategies. Developing a post-market surveillance plan, engaging with healthcare professionals, and conducting regular audits are vital in maintaining device safety and effectiveness, ensuring adherence to regulatory standards.
In conclusion, the regulatory framework in Argentina presents both challenges and opportunities for medical device manufacturers. By equipping themselves with the right knowledge and strategies, organizations can adeptly navigate this complex environment and ensure their innovative solutions positively impact the healthcare sector. With diligent preparation and proactive compliance efforts, manufacturers can thrive in this dynamic market.
Frequently Asked Questions
What is the main governing body for healthcare instrument approval in Argentina?
The National Administration of Drugs, Food and Medical Devices (ANMAT) is the principal governing body overseeing regulatory pathways for healthcare instrument approval in Argentina.
Why is it important to understand the regulatory pathways for approval in Argentina?
Familiarity with the regulatory pathways is essential because ANMAT evaluates and approves health-related products, and understanding these pathways helps ensure compliance with the relevant legislation.
How are medical devices classified in Argentina?
Medical devices in Argentina are classified into four categories (I, II, III, IV) based on their risk levels, which directly influences the regulatory pathways for approval and the corresponding requirements.
What recent changes are occurring in the compliance landscape for medical device approvals in Argentina?
ANMAT is streamlining regulatory pathways to enhance efficiency, which can result in expedited approvals and reflects a growing commitment to fostering innovation in the healthcare technology sector.
What role do organizations like bioaccess® play in the regulatory approval process?
Organizations like bioaccess® assist Medtech startups in understanding the regulatory pathways for approval in Argentina, facilitating the progression of medical devices from early-feasibility studies to commercialization.
What types of studies does bioaccess® oversee?
bioaccess® oversees various studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).
How has technology improved the application preparation process for regulatory compliance?
Tools like RegDesk have significantly reduced the application preparation duration from six months to just six days, aiding compliance initiatives in the regulatory process.
Why is it crucial to stay informed about ANMAT's guidelines?
Staying informed about ANMAT's guidelines is crucial because any changes can significantly impact the submission process for healthcare instruments.




