Overview
Navigating clinical research in Latin America involves understanding the region's unique regulatory frameworks, diverse patient populations, and the strategic advantages of conducting studies there, such as lower costs and enhanced recruitment opportunities. The article highlights how partnerships with local stakeholders and the integration of technology are essential for overcoming challenges and optimizing the research process, ultimately positioning Latin America as a burgeoning hub for clinical trials.
Introduction
Latin America is rapidly establishing itself as a burgeoning hub for clinical research, characterized by a unique blend of opportunities and challenges. With its diverse populations, the region offers a rich tapestry for studying various health outcomes, making research findings more relevant and impactful.
However, the journey is not without hurdles; navigating the intricate regulatory frameworks and fragmented healthcare systems across countries like Brazil, Mexico, and Argentina can be daunting. As the clinical trials market in Latin America is projected to soar to USD 2,654.0 million by 2030, understanding the dynamics of this landscape becomes crucial for researchers aiming to optimize trials, enhance patient recruitment, and ensure data reliability.
From leveraging cost-effective solutions to building strategic partnerships, the potential for growth and innovation in Latin American clinical research is immense, setting the stage for a transformative future in healthcare advancements.
Overview of Clinical Research Landscape in Latin America
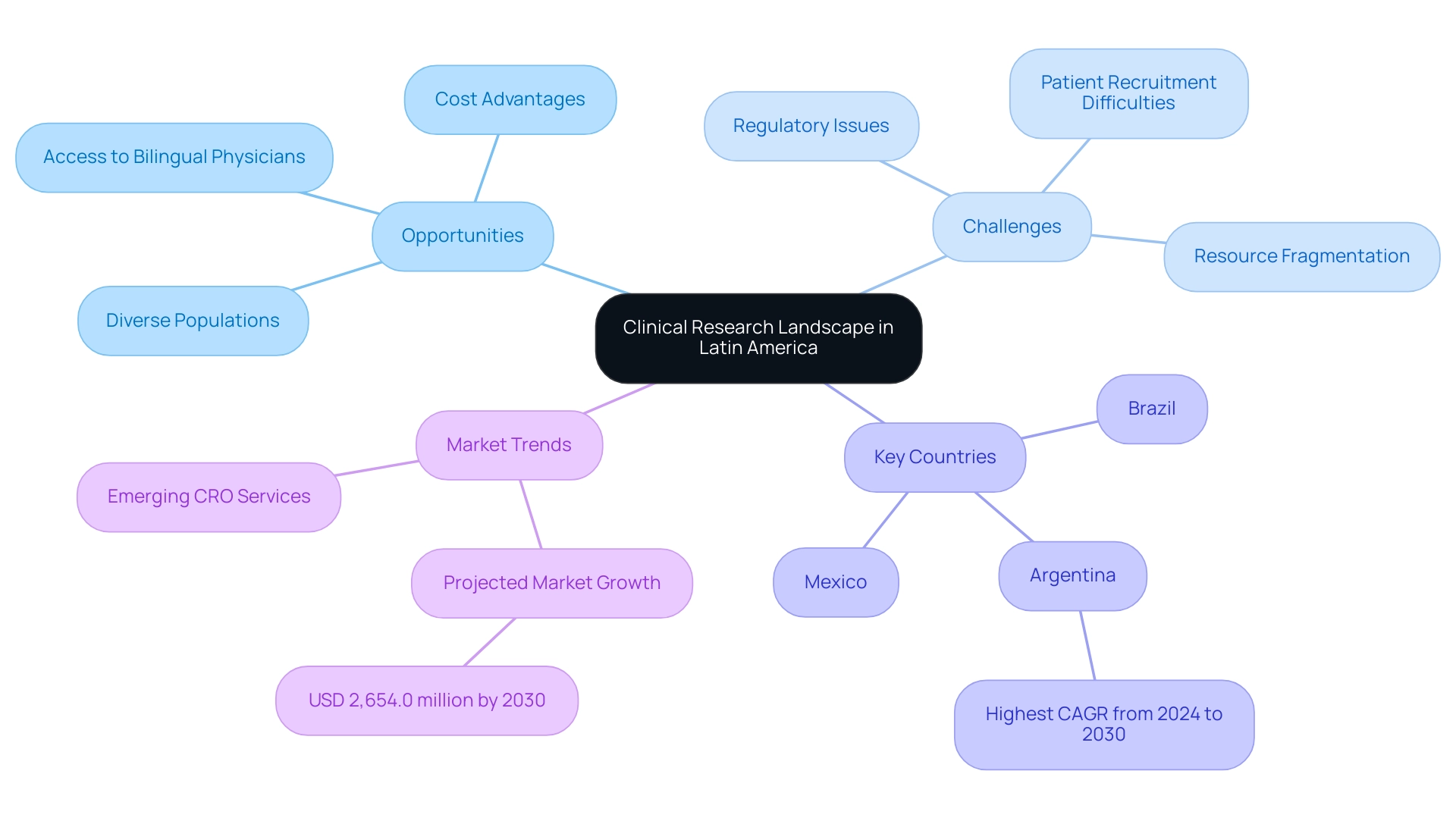
Latin America is swiftly becoming a central hub for medical studies, presenting a varied and dynamic environment brimming with both prospects and challenges. The region's diverse populations offer a rich context for exploring a multitude of health outcomes, enhancing the significance of findings. However, navigating clinical research in Latin America involves addressing the complex regulatory frameworks and healthcare systems unique to each country, which presents notable challenges, particularly the fragmentation of resources and the lack of established CRO corporate structures.
Nations like Brazil, Mexico, and Argentina are especially attractive for research studies, due to their growing infrastructure and strong dedication to advancing medical research. Significantly, the partnership between Greenlight Guru and bioaccess® seeks to optimize the research process by tackling these obstacles, including professionalism and language differences, while also utilizing the historical benefits of carrying out studies in South America, such as access to bilingual U.S. board-certified physicians and reduced study expenses. The research studies market in Latin America is anticipated to reach USD 2,654.0 million by 2030, emphasizing the considerable growth potential in this sector.
Recent trends suggest that Argentina is anticipated to record the highest compound annual growth rate (CAGR) from 2024 to 2030, highlighting its potential as a center for research studies. With bioaccess® at the forefront of delivering affordable, high-quality CRO services, researchers who grasp these dynamics are better prepared for navigating clinical research in Latin America, enhancing their studies, boosting patient recruitment, and guaranteeing the dependability of their data, which is vital for attaining definitive outcomes in the changing landscape of medical research.
Benefits of Conducting Clinical Trials in Latin America
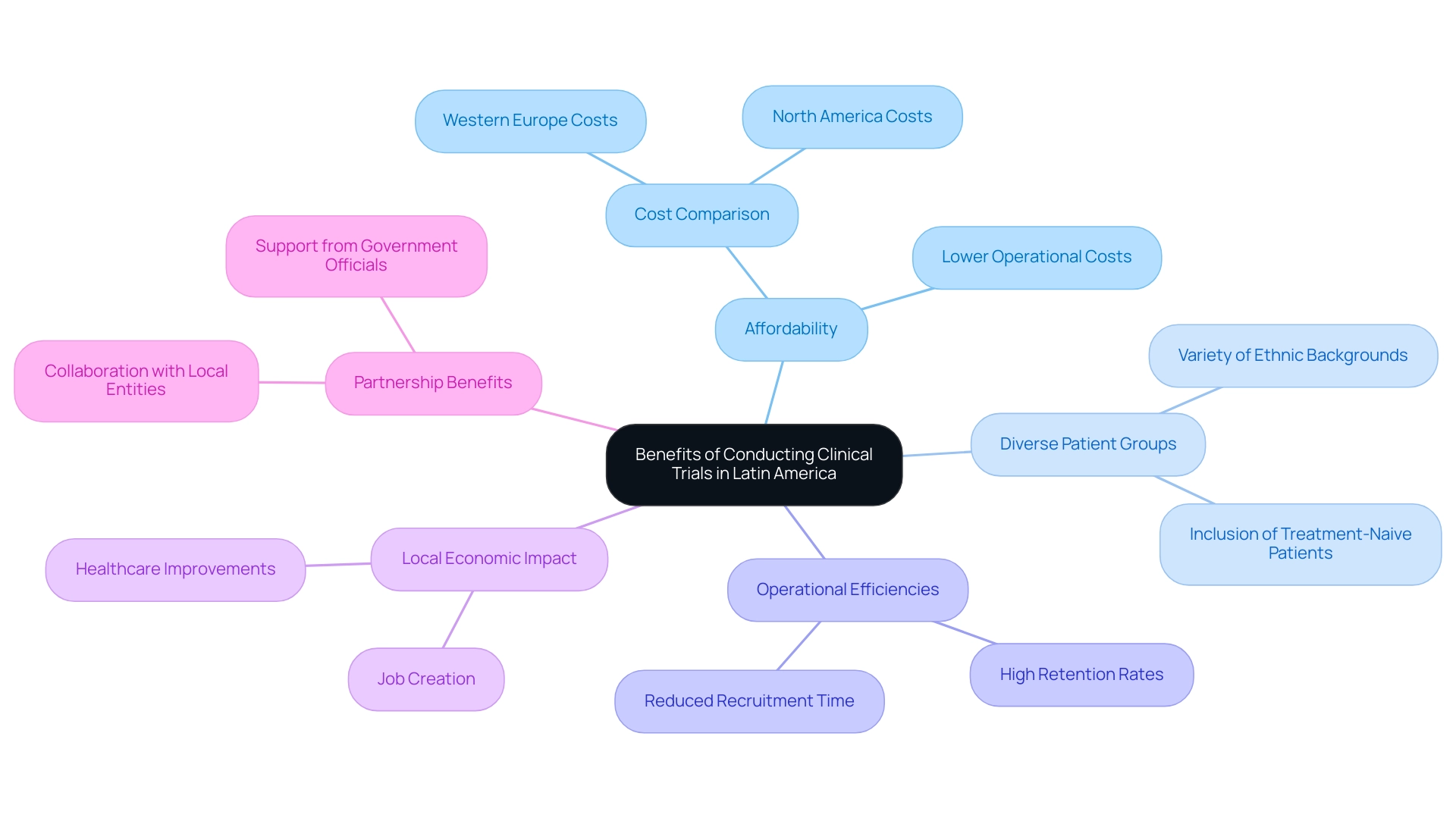
Navigating clinical research in Latin America offers numerous benefits, particularly in carrying out medical studies in South America, such as affordability and access to varied patient groups. The operational costs in this region are significantly lower than those in North America, where the average cost per participant ranges from $15,000 to $25,000, and in Western Europe, where costs also average between $15,000 and $25,000. This financial advantage makes South America an attractive location for pharmaceutical firms and sponsors navigating clinical research in Latin America while looking to enhance their budget allocations.
Furthermore, the region's rich tapestry of ethnic and socioeconomic backgrounds enhances the data collected, bolstering the generalizability of study outcomes. The inclusion of treatment-naive patients—those who have not yet been exposed to investigational therapies—can lead to more reliable results, as their responses are less influenced by prior treatment experiences. As highlighted by industry expert Nana Twum-Danso, 'The authors would like to thank the members of the 2024 advisory panel for their input in meetings and reviews of drafts.'
This variety present in South American studies not only enhances the academic environment but also corresponds with increasing demands for fairness in healthcare, tackling both patient experiences and doctor views on care. Furthermore, the partnership between bioaccess™ and Caribbean Health Group intends to establish Barranquilla as the most appealing location for navigating clinical research in Latin America, backed by Colombia's Minister of Health, increasing the region's allure. This collaboration has resulted in substantial enhancements in research services, accomplishing over a 50% decrease in recruitment time and sustaining a 95% retention rate.
Furthermore, media attention by Clinical Leader has emphasized the increasing importance of navigating clinical research in Latin America, further strengthening the area's reputation. A case study on key cost drivers in the U.S. reveals that labor costs, site management, and patient recruitment significantly contribute to higher expenses, further illustrating the financial benefits of conducting studies in South America. This multifaceted approach positions the region as a strategic choice for sponsors navigating clinical research in Latin America, as they seek both quality and cost-effectiveness in their research endeavors, while also contributing to local economies through job creation and healthcare improvements.
Understanding Regulatory Frameworks for Clinical Trials
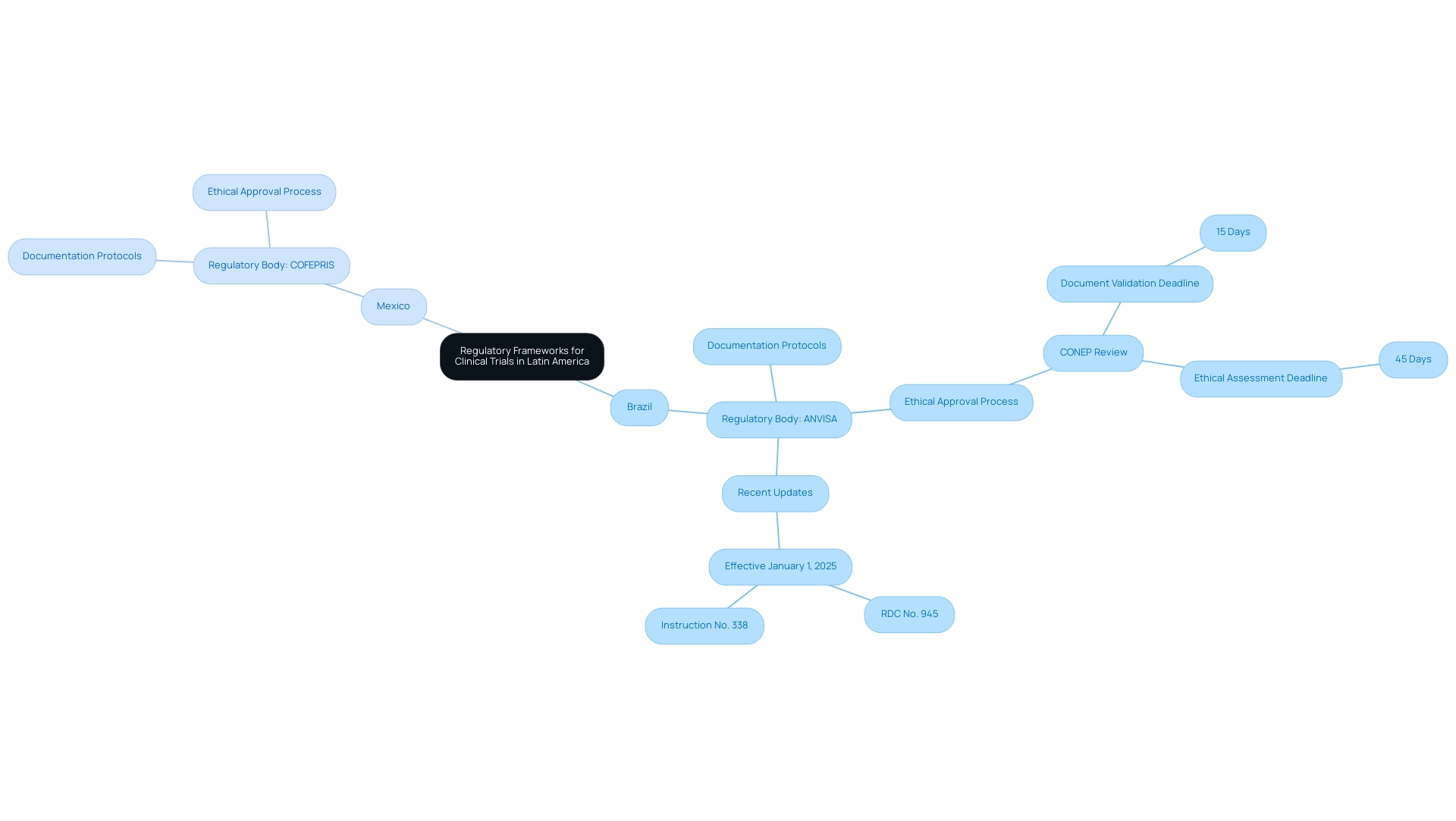
Navigating clinical research in Latin America requires a nuanced understanding of each nation's unique regulatory environment for research studies. As a prominent Contract Research Organization (CRO), bioaccess® is dedicated to supporting medical device studies throughout the region, ensuring adherence to local regulations. In Brazil, the National Health Surveillance Agency (ANVISA) supervises research studies, while in Mexico, the Federal Commission for Protection against Health Risks (COFEPRIS) possesses this authority.
Each regulatory body has established specific documentation protocols, ethical approval processes, and submission timelines that researchers must follow to ensure compliance. For instance, in Brazil, projects requiring review by the National Research Ethics Commission (CONEP) must adhere to strict timelines, with:
- A 15-day deadline for document validation
- 45 days for ethical assessments
Additionally, it is critical to note that requests for new registration by supporting institutions are prohibited within a period of 12 months after cancellation, which can significantly impact management of the study.
Researchers must also be aware of the recent update to ANVISA's research regulations, effective January 1, 2025, which revokes previous resolutions and emphasizes enhanced ethical oversight. The ethical standards promoted by Brazil’s National Research Ethics Commission safeguard the rights of research participants, including their autonomy, culture, beliefs, and values. As emphasized by Kendle, a CRO specializing in research management, average approval durations in the region can range from 14 to 16 weeks, making it essential for researchers to understand these timelines and requirements to expedite the approval process.
The recent case study on ANVISA's Clinical Trials Regulation Update illustrates the ongoing evolution of the regulatory framework in Brazil, emphasizing that navigating clinical research in Latin America is essential for efficient study management and compliance, ultimately facilitating successful research studies across South America. Furthermore, if you have any queries or concerns about the processing of your information, you may contact our Grievance Officer at IMH ASSETS CORP (doing business as 'bioaccess®'), ensuring that your data protection rights are respected. Moreover, perspectives from industry experts highlight the significance of grasping local market dynamics and fostering connections with regional stakeholders, which can greatly improve the success of studies in the area.
Challenges in Clinical Research: Recruitment and Logistics
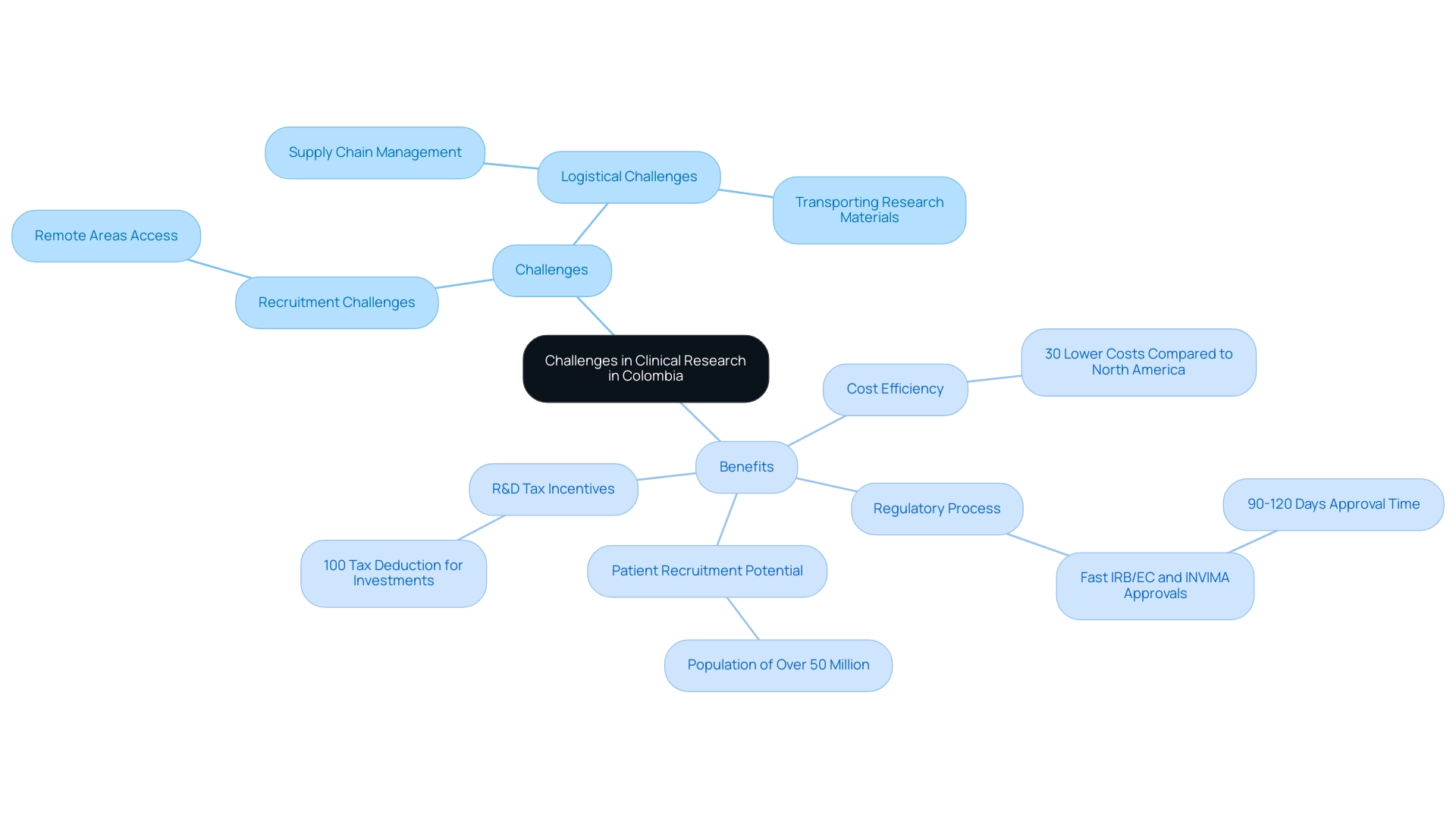
Recruitment and logistical challenges are significant factors when navigating clinical research in Latin America. However, Colombia offers significant competitive benefits for first-in-human studies. The country offers a cost efficiency of over 30% compared to North America and Western Europe, along with a rapid regulatory process, where IRB/EC and INVIMA approvals can be secured in just 90-120 days.
With a healthcare system ranked among the best globally, and a population of over 50 million, the potential for patient recruitment is robust despite challenges in remote areas with limited access. Logistical challenges, such as supply chain management and transporting research materials, remain obstacles; yet, local collaborations are essential in overcoming these hurdles. The presence of 4,232 Contract Research Organizations (CROs) in the U.S. underscores the significance of navigating clinical research in Latin America to enhance research capabilities.
For instance, Parexel's partnership with local CROs and ABRACRO demonstrates how utilizing local knowledge can optimize logistics and guarantee adherence to regulatory standards, ultimately enabling more effective studies. Additionally, Colombia offers significant R&D tax and financial incentives, including a 100% tax deduction for investments in science, technology, and innovation projects. New legislation in Brazil, such as Law 14.874/24, aims to improve the evaluation process for research studies, which is essential for navigating clinical research in Latin America and enhancing healthcare practices and access to new therapies.
Moreover, hospitals in Colombia must undergo a strict ICH/GCP certification procedure before carrying out medical studies, ensuring high-quality standards in studies. This extensive structure of assistance and incentives establishes Colombia as a top location for medical studies.
Building Partnerships: Collaborating with Local Stakeholders
Creating strong collaborations with local stakeholders—including healthcare providers, regulatory agencies, and patient advocacy groups—is essential for navigating clinical research in Latin America successfully. Dushyanth Surakanti, Founder and CEO of Sparta Biomedical, highlights the significance of these partnerships, stating, "Our experience with bioaccess® during its initial human evaluation in Colombia emphasized the importance of collaborating with local associates to navigate the challenges of medical studies efficiently." The recent endorsement of Law 14.874/24 in May 2024 further emphasizes the significance of these alliances, as it simplifies the evaluation process for research in Brazil, which is critical for navigating clinical research in Latin America.
Engaging local investigators not only enhances the understanding of patient populations but also leverages their networks to improve recruitment strategies. As professor J Jaime Miranda pointed out, "These alliances and their outputs will be crucial for strengthening health systems dealing with major public health challenges." Additionally, insights from Dr. John B. Simpson concerning Avinger's OCT-guided atherectomy studies in Cali, Colombia, underscore the collaborative spirit with LATAM CRO specialists, emphasizing that navigating clinical research in Latin America highlights the influence of thorough study management services, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project management
- Reporting
The INVIMA, Colombia's National Food and Drug Surveillance Institute, plays a critical role as a Level 4 health authority by PAHO/WHO, overseeing medical device regulations and ensuring compliance with safety standards. The case studies on the growth of medtech trials in Colombia and Paraguay illustrate the significant impact of these partnerships on local economies, job creation, economic growth, healthcare improvement, and international collaboration. By fostering trust and maintaining open lines of communication with local stakeholders, researchers can create a collaborative environment that significantly enhances study outcomes and boosts participant satisfaction.
Leveraging Technology for Enhanced Clinical Research
Navigating clinical research in Latin America relies heavily on the incorporation of technology to change medical studies. Tools such as electronic data capture systems, telemedicine, and mobile health applications are revolutionizing how studies are conducted. Navigating clinical research in Latin America involves innovations that streamline processes and significantly improve data management and patient engagement.
Telemedicine, for instance, facilitates remote monitoring of participants, effectively minimizing travel requirements and enhancing accessibility for diverse populations. Gustavo Werutsky highlights the importance of these advancements, stating,
The academic Cancer Research Groups operating in this area have a crucial role in training medical oncologists and study specialists in proposing investigator-initiated studies assessing topics with epidemiological significance for this region.
This training is essential as it equips professionals for navigating clinical research in Latin America and effectively utilizing new technologies.
With approximately 65% of revenue derived from competitive and market intelligence teams, researchers must remain vigilant about the latest technological trends to seamlessly incorporate these innovations into their study designs. Moreover, bioaccess® demonstrates the potential of these advancements, providing extensive study management services including feasibility assessments, site selection, compliance reviews, and project management. Their expertise in executing Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies ensures that Medtech startups receive crucial support in navigating regulatory approval, patient recruitment, and timely data delivery.
Navigating clinical research in Latin America, the historical data for the clinical study technology and services market from 2018 to 2023 illustrates a steady growth trajectory, with the region projected to account for 9.3% of the global share by 2024. This growth is driven by increased demand for efficient testing processes and enhanced infrastructure. By utilizing telemedicine and other technological innovations through expert collaborations like bioaccess®, researchers can anticipate enhanced participation rates and overall study effectiveness.
Future Trends and Opportunities in Latin American Clinical Research
Navigating clinical research in Latin America is on the verge of change, propelled by various emerging trends that are set to reshape the domain. A significant rise in funding for healthcare infrastructure is creating opportunities for more resilient clinical studies, while the growing awareness among patients regarding their involvement in investigations is encouraging a more involved participant base. Furthermore, advancements in digital health technologies are facilitating efficient data collection and management processes.
The rise of patient-centric study models is particularly noteworthy as it emphasizes participant engagement and the quality of data collected, essential for successful outcomes in the region. Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, emphasizes the critical role of local expertise in navigating these changes, stating,
Local knowledge is not just beneficial; it’s essential for the success of our studies in the region.
This emphasizes the urgent need for Medtech companies to work together effectively, bridging gaps in research and innovation.
Moreover, thorough management services for research studies, including feasibility assessments, site selection, compliance reviews, setup, import permits, project management, and reporting, are essential in tackling the complex challenges encountered in this sector. For example, meticulous experiment setup and project management ensure that studies align with local regulations, while thorough compliance reviews safeguard ethical standards. Anil Kumar P., Research Manager in Healthcare, underscores this commitment, stating,
I am committed to staying at the forefront of industry innovations, ensuring that my work consistently exceeds client expectations.
Furthermore, tackling logistical challenges, like language barriers, is essential for ethical treatment and participant involvement in research studies. To effectively bridge the gap between innovation and execution, researchers must adopt a solution-driven approach that embraces these trends. By remaining informed and flexible, clinical researchers can focus on navigating clinical research in Latin America, leveraging the evolving landscape of clinical trials to drive economic growth, job creation, and international collaboration.
Conclusion
Latin America is poised to become a pivotal player in the global clinical research arena, characterized by a unique blend of advantages and challenges. The region's diverse populations not only enhance the relevance of research findings but also present a wealth of opportunities for pharmaceutical companies and sponsors. With the projected clinical trials market reaching USD 2,654 million by 2030, the incentive to invest in this burgeoning landscape is clear. Cost-effectiveness, access to treatment-naïve patients, and a commitment to improving healthcare infrastructure position Latin America as an attractive destination for clinical trials.
However, navigating the regulatory frameworks and overcoming logistical hurdles remain critical for success. Researchers must familiarize themselves with the specific requirements of each country, ensuring compliance with local regulations to streamline the approval process. Building strong partnerships with local stakeholders is essential, as these collaborations can enhance recruitment strategies and foster trust within communities. The integration of technology, from telemedicine to electronic data capture systems, further streamlines clinical research processes, improving data management and patient engagement.
As the region embraces emerging trends such as patient-centric research models and digital health technologies, the future of clinical research in Latin America looks promising. By leveraging local expertise and innovative solutions, researchers can not only drive successful outcomes but also contribute to the overall enhancement of healthcare systems. The potential for economic growth, job creation, and international collaboration in Latin America underscores the importance of this evolving landscape, marking a transformative era for clinical trials and healthcare advancements.
Frequently Asked Questions
Why is Latin America becoming a central hub for medical studies?
Latin America is becoming a central hub for medical studies due to its diverse populations, which provide a rich context for exploring various health outcomes, and the growing infrastructure and commitment to advancing medical research in countries like Brazil, Mexico, and Argentina.
What are some challenges associated with clinical research in Latin America?
Challenges include navigating complex regulatory frameworks, fragmented resources, and the lack of established Contract Research Organization (CRO) structures unique to each country.
What advantages does conducting research in Latin America offer?
Advantages include lower operational costs compared to North America and Western Europe, access to varied patient groups, and the inclusion of treatment-naive patients, which enhances the reliability of study results.
What is the expected growth of the research studies market in Latin America by 2030?
The research studies market in Latin America is anticipated to reach USD 2,654.0 million by 2030, indicating significant growth potential in this sector.
Which country in Latin America is expected to have the highest growth rate for research studies from 2024 to 2030?
Argentina is expected to record the highest compound annual growth rate (CAGR) from 2024 to 2030, highlighting its potential as a center for research studies.
How does the partnership between Greenlight Guru and bioaccess® aim to improve clinical research in Latin America?
The partnership aims to optimize the research process by addressing challenges such as professionalism and language differences while leveraging the benefits of conducting studies in South America, including access to bilingual U.S. board-certified physicians and reduced study expenses.
What impact does diversity in patient demographics have on clinical research in Latin America?
The diversity in ethnic and socioeconomic backgrounds enhances data collected, improving the generalizability of study outcomes and enabling more reliable results, especially with treatment-naive patients.
How has the partnership between bioaccess™ and Caribbean Health Group influenced clinical research in Colombia?
This partnership has established Barranquilla as a desirable location for clinical research, resulting in significant improvements in research services, including over a 50% decrease in recruitment time and a 95% retention rate.
What financial benefits do sponsors gain from conducting studies in South America?
Sponsors benefit from significantly lower costs per participant compared to North America and Western Europe, which can range from $15,000 to $25,000, making South America an attractive option for budget-conscious research.
How does conducting clinical research in Latin America contribute to local economies?
Conducting research in Latin America contributes to local economies through job creation and improvements in healthcare services.

