Overview
Navigating regulations in Latin America is essential for Medtech companies, given the complex and varied legal landscape across different countries. INVIMA in Colombia serves as a key regulatory body in this context. Understanding local regulations, engaging with regional experts, and forming strategic partnerships are crucial for successful market entry and compliance. These strategies can significantly reduce approval times and enhance operational efficiency, underscoring the importance of collaboration in this intricate environment.
Introduction
In the intricate world of medical technology, navigating the regulatory landscape of Latin America presents a formidable challenge that demands a nuanced understanding of diverse laws and local practices. Each country introduces its own set of regulations and authorities, compelling Medtech companies to tread carefully to ensure compliance and streamline market entry.
Colombia, with its prominent regulatory body INVIMA, exemplifies the complexities that lie ahead for businesses aiming to introduce innovative medical devices. As the Medtech sector evolves, emerging partnerships and strategic collaborations become essential for overcoming hurdles such as recruitment delays and regulatory discrepancies.
By leveraging local expertise and forging strong connections, companies can significantly enhance their chances of success in this dynamic market, ultimately improving healthcare outcomes across the region.
Understanding the Regulatory Landscape in Latin America
Navigating regulations in Latin America, particularly in Colombia, presents a complex mosaic of laws that vary significantly across countries. Each nation is governed by its own regulatory body, with INVIMA in Colombia serving as a prominent figure in medical device oversight, classified as a Level 4 health authority by PAHO/WHO. For Medtech firms aiming to enter these markets, understanding these distinctions and navigating regulations in Latin America is crucial, especially with new partnerships like bioaccess™ collaborating with Welwaze Medical Inc. to support the introduction of the Celbrea® medical device in Colombia.
As we look toward 2025, the number of oversight bodies managing medical devices in Latin America continues to rise, underscoring the growing significance of adherence within the Medtech industry. Engaging with local experts, such as Katherine Ruiz, an authority in Affairs for Medical Devices and In Vitro Diagnostics, is essential, as they provide valuable insights into the specific nuances that may impact product approval timelines and market strategies. A recent case study further emphasizes this need, showcasing that GlobalCare Clinical Trials, in partnership with bioaccess™, achieved over a 50% reduction in recruitment time and 95% retention rates through effective management and compliance strategies.
bioaccess™ offers a comprehensive range of clinical trial management services, including:
- feasibility studies
- site selection
- compliance reviews
- trial setup
- import permits
- project management
- reporting
Furthermore, as health system leaders increasingly emphasize enhancing consumer experience and involvement—almost three-quarters of them in 2025—navigating regulations in Latin America becomes even more essential. Virtual health and alternative care locations are emerging as effective methods to improve accessibility and decrease patient wait times, further impacting oversight considerations. Alicia Janisch, Vice Chair and US Health Care Sector Leader at Deloitte, notes that medical technology firms must keep pace with changing consumer preferences and expectations.
By staying informed about the current legal framework and leveraging expert opinions, medical technology firms can develop successful market entry strategies tailored to the unique challenges of each country in Latin America while addressing broader challenges such as the anticipated impact of climate change on health systems.
Key Regulatory Requirements for Medtech Companies
Navigating regulations in Latin America is essential for Medtech companies to adhere to several critical compliance requirements, pivotal for successful product development and commercialization. These include:
- Product Classification: A thorough understanding of product classification according to risk levels—Class I, II, and III—is essential. This classification not only determines the oversight pathway but also affects the necessary documentation and approval processes. In 2025, the landscape for product classification is evolving, with increased scrutiny on software as a medical device (SaMD), as the FDA is likely to introduce more stringent requirements for SaMD, highlighting the implications of cybersecurity risks, as emphasized by recent FDA guidelines.
- Clinical Trials: Obtaining approval for clinical trials is a fundamental step that involves regional regulatory bodies. Companies must prepare detailed study protocols and obtain ethical approvals, which can vary significantly across countries. Successful clinical trial approvals in Latin America have demonstrated that navigating regulations in the region with tailored approaches can expedite the process, allowing for faster entry into the market. For example, bioaccess® has effectively assisted Medtech startups by managing feasibility studies, site selection, and review processes, demonstrating how valuable guidance can result in successful outcomes. Their comprehensive services also include trial set-up, project management, and reporting, ensuring that all aspects of the clinical trials are handled efficiently.
- Quality Management Systems: Implementing robust quality management systems that comply with ISO 13485 standards is crucial. These systems ensure that products meet stringent safety and efficacy requirements, increasingly emphasized by regulatory authorities. Companies that prioritize quality management not only enhance their compliance but also build trust with stakeholders and consumers.
- Labeling and Advertising: Adherence to regional language requirements for product labeling is mandatory, and all labels must include essential safety information. Furthermore, advertising practices must align with local regulations to prevent misleading claims. As the oversight environment tightens, particularly concerning digital health and connected devices, adherence to these guidelines is more important than ever. The FDA Cybersecurity Guidance (2023) requires manufacturers to provide a cybersecurity risk management plan, while the EU MDR mandates rigorous testing of connected device security throughout the product lifecycle. Furthermore, Gartner forecasts that by 2025, a third of all nations will have legislation specifically targeting ransomware payments, highlighting the increasing cybersecurity issues that healthcare technology firms must manage.
By comprehending and maneuvering through these compliance necessities, healthcare technology companies can effectively position themselves for success in the Latin American market, especially by navigating regulations in the region to leverage its unique opportunities while mitigating potential risks. Through the expertise of bioaccess®, companies can ensure that they are well-prepared to meet these challenges and capitalize on the growth potential in Latin America.
Challenges in Navigating Regulatory Compliance
Navigating regulatory compliance in Latin America presents significant challenges for Medtech companies, particularly in 2025. The regulatory landscape is characterized by a lack of harmonization, requiring companies to manage a patchwork of rules that vary significantly from one country to another. This complexity often results in increased time and resources devoted to regulations, hindering the speed at which innovative medical technologies can reach the market. bioaccess® offers comprehensive clinical trial management services, including feasibility assessments and compliance reviews, simplifying this process and ensuring efficient navigation through regulatory requirements.
Recruitment Issues: Securing qualified clinical research sites and investigators remains a critical hurdle. Many regions face a shortage of experienced professionals familiar with specific medical technologies, leading to delays in clinical trials. Statistics indicate that recruitment challenges can result in trial delays of up to 30%, underscoring the necessity for strategic planning and community partnerships. bioaccess® supports Medtech startups with cost-effective, high-quality clinical research services, including site feasibility assessments, investigator selection, and project management, which can help mitigate these recruitment issues.
Language differences can complicate interactions with governing authorities and community stakeholders. Miscommunications may lead to misunderstandings that stall the approval process, emphasizing the importance of employing bilingual professionals or translation services to facilitate clear communication.
Cultural Differences: A nuanced understanding of local business practices and cultural dynamics is essential for fostering effective partnerships. Companies that invest time in building relationships and understanding regional customs are more likely to excel in navigating regulations in Latin America successfully. As indicated by industry leaders, adapting to these cultural contexts can significantly enhance collaboration and adherence efforts.
Emerging Cybersecurity Concerns: With predictions that cybercrime will cost $10.5 trillion annually by 2025, medical technology firms must also address new cybersecurity regulations. Governments are introducing stringent requirements for medical device cybersecurity, adding another layer of complexity to compliance.
In light of these challenges, Medtech companies are increasingly adopting innovative strategies to overcome compliance obstacles. For instance, some organizations leverage technology to streamline the submission process, while others form alliances with local firms to enhance recruitment efforts. As Monica Mabel Guaita, CEO and Founding Partner of MMGC SRL, observes, "Currently, several Latin American oversight agencies are gradually migrating from long and heavy paper-based procedures to online submissions for medical device registrations and license renewals."
This transition reflects the evolving governance frameworks aimed at attracting innovation while ensuring safety. Companies that proactively address these challenges, leveraging bioaccess's expertise in trial setup, monitoring, and reporting, will be better positioned to thrive in the Latin American market.
Strategies for Successful Regulatory Navigation
Successfully navigating the regulatory landscape in Latin America requires Medtech companies to implement several key strategies:
- Engage Regional Experts: Collaborating with regional consultants is vital, as they provide crucial insights into the specific requirements and processes unique to each nation, including the feasibility and selection of research sites and principal investigators (PIs). Their expertise can significantly mitigate the risk of regulatory issues and streamline the approval process. As emphasized by April Chan-Tsui, Director of Product Operations at Clarivate, involving local specialists is essential for guaranteeing adherence in a complex legal environment.
- Develop a Comprehensive Compliance Strategy: Companies should formulate a detailed compliance strategy that clearly outlines the necessary steps for adherence, including the review and feedback on study documents to meet country requirements. This strategy must encompass timelines, resource allocation, and a proactive approach to addressing evolving compliance standards, ensuring market readiness and minimizing delays. With over 15 years of experience in the Medtech sector, bioaccess® understands the importance of such strategies in facilitating successful market entry.
- Build Relationships with Oversight Authorities: Establishing strong connections with nearby governing bodies, such as INVIMA, is vital. INVIMA oversees medical device matters and ensures adherence to health standards, which can facilitate smoother communication, foster trust, and expedite the approval process. These relationships ultimately lead to faster market entry for innovative medical devices. Additionally, bioaccess offers services to assist in obtaining the necessary import permits and nationalization of investigational devices, further streamlining the approval process.
- Invest in Training and Development: Providing continuous education for personnel on regional rules and adherence necessities enhances the organization’s ability to navigate the legal framework efficiently. A well-informed team is better equipped to adapt to changes and ensure adherence to local standards, including the necessary IRB/EC approvals and INVIMA regulations.
- Leverage Technology: The integration of artificial intelligence (AI) and automation in Regulatory Affairs is transforming how companies handle adherence. As illustrated in the case study titled "Future of Regulatory Affairs with AI and Automation," these technologies enhance efficiency and decision-making, enabling organizations to better navigate complex global requirements while prioritizing patient safety and innovation.
- Prioritize Post-Market Surveillance: Ongoing observation of medical devices after approval is vital for upholding regulations and ensuring patient safety. Companies should establish robust post-market surveillance systems to address any emerging issues promptly, in line with INVIMA's oversight responsibilities. This encompasses regular updates on study status, inventory, and any significant and minor adverse events to ensure ongoing adherence.
By implementing these strategies, medical technology firms can effectively focus on navigating regulations in Latin America, ensuring adherence and accelerating the journey to market for their innovative medical devices. Furthermore, Colombia's competitive advantages, such as cost efficiency, rapid processes, high-quality healthcare, and R&D tax incentives, provide an additional layer of support for successful first-in-human clinical trials.
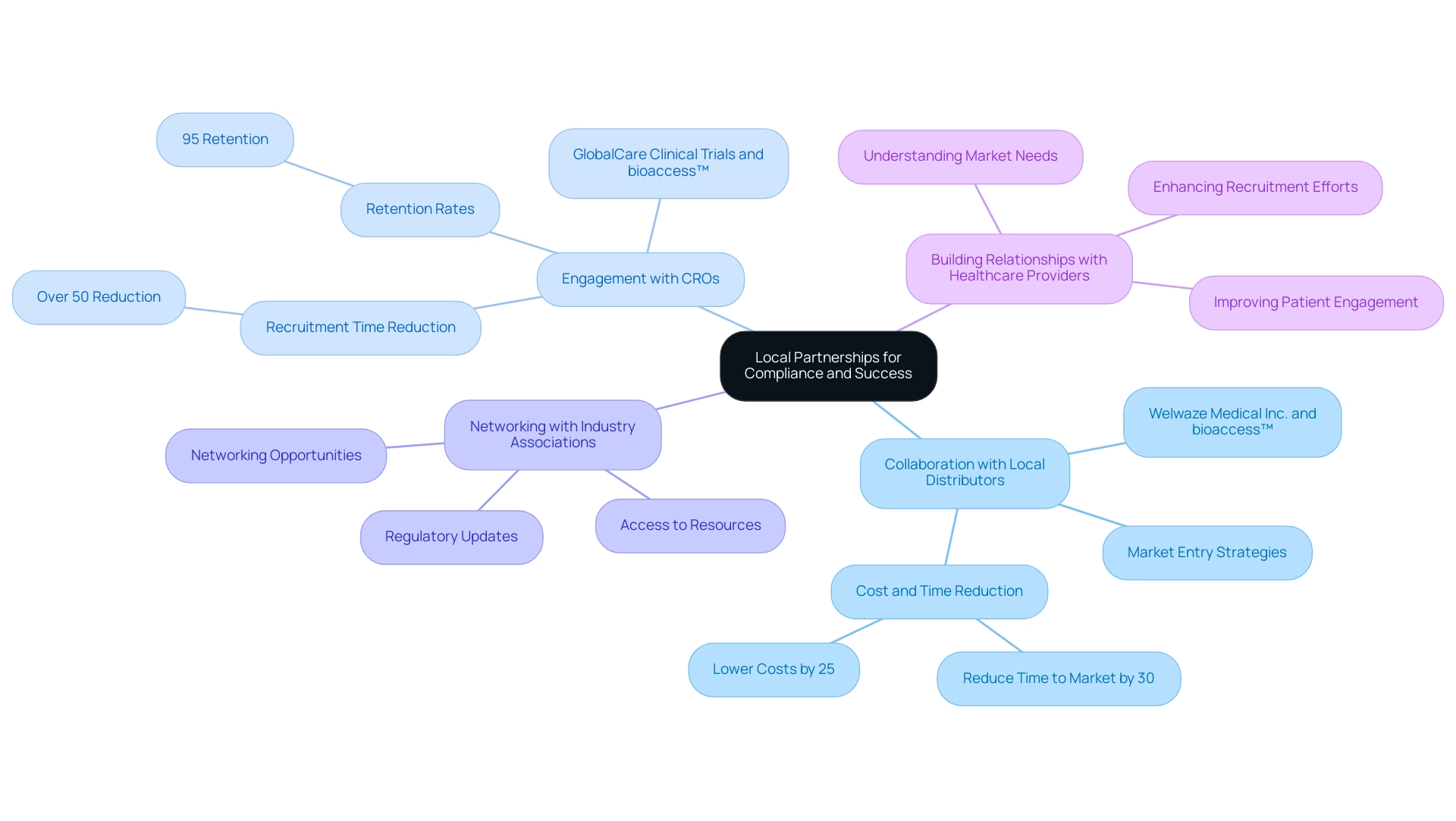
Leveraging Local Partnerships for Compliance and Success
Local partnerships are pivotal for Medtech companies aiming to thrive in the Latin American market. These collaborations can manifest in several impactful ways:
- Collaboration with Local Distributors: Engaging with established local distributors offers invaluable insights into market dynamics and compliance landscapes, significantly easing the product launch process. For instance, Welwaze Medical Inc. chose bioaccess™ as its compliance and market access consulting firm to facilitate the launch of the Cerebral® medical device in Colombia. Such partnerships enhance market entry strategies and align with the unique needs of the region. As highlighted in a case study on standardization for MedTech collaboration, aligning rules can reduce time to market by up to 30% and lower costs by approximately 25%, facilitating smoother processes.
- Engagement with Clinical Research Organizations (CROs): Collaborating with CROs that have regional expertise can simplify the clinical trial process. GlobalCare Clinical Trials' collaboration with bioaccess™ exemplifies this, leading to an impressive reduction of over 50% in recruitment time and 95% retention rates, enhancing the efficiency of clinical trials in Colombia. These organizations excel at navigating regulations in Latin America, ensuring adherence to compliance standards and efficiency throughout the study phases. bioaccess™ specializes in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).
- Networking with Industry Associations: Joining regional industry associations offers access to essential resources, networking opportunities, and timely updates on regulatory changes. This engagement fosters a collaborative environment that can lead to shared knowledge and best practices among Medtech companies.
- Building Relationships with Healthcare Providers: Establishing strong connections with healthcare providers in the area is crucial for understanding market needs and enhancing recruitment efforts for clinical trials. These relationships can lead to better patient engagement and improved outcomes, ultimately benefiting both the companies and the communities they serve.
The importance of these local partnerships cannot be overstated. They facilitate smoother operations and contribute to the overall success of healthcare initiatives in the region. As Jaime Miranda, a research professor, noted, "These alliances and their outputs will be crucial for strengthening health systems dealing with major public health challenges."
Moreover, with 13% of Latino-owned small enterprises facing failure within their first year, collaboration becomes essential in overcoming obstacles to success in the medical technology sector. By fostering these alliances, medical technology firms can enhance their market presence and drive innovation, ultimately improving patient access to groundbreaking medical solutions. Additionally, the impact of Medtech clinical studies on local economies, including job creation and healthcare improvement, underscores the critical role of local partnerships in navigating regulations in Latin America.
Conclusion
The regulatory landscape in Latin America, particularly in Colombia, presents both significant challenges and opportunities for Medtech companies. Understanding the diverse regulations and engaging local expertise, such as INVIMA's oversight, is essential for successful market entry. Forming partnerships with local distributors and clinical research organizations can streamline the approval process and enhance recruitment efforts, ultimately facilitating faster access to innovative medical devices.
Navigating the complexities of product classification, clinical trials, and quality management systems is critical for compliance. Companies that invest in training, leverage technology, and establish strong relationships with regulatory authorities will be better positioned to overcome these challenges. The importance of local partnerships cannot be overstated; they provide valuable insights into market dynamics and foster collaboration that can lead to improved healthcare outcomes.
As the Medtech sector in Latin America continues to evolve, companies that proactively address regulatory hurdles and embrace strategic collaborations will thrive. By doing so, they not only enhance their own success but also contribute to the advancement of healthcare systems across the region, ultimately improving patient access to life-saving technologies.
Frequently Asked Questions
What are the key regulatory bodies involved in medical device oversight in Latin America?
Each country in Latin America has its own regulatory body, with INVIMA in Colombia being a prominent figure, classified as a Level 4 health authority by PAHO/WHO.
Why is understanding regulations in Latin America important for Medtech firms?
Understanding the distinct regulations across Latin American countries is crucial for Medtech firms to navigate compliance requirements effectively, which is essential for successful product development and commercialization.
What specific compliance requirements must Medtech companies adhere to in Latin America?
Medtech companies must adhere to several compliance requirements, including product classification, clinical trials approval, quality management systems, and labeling and advertising regulations.
How does product classification impact the regulatory process?
Product classification according to risk levels (Class I, II, and III) determines the oversight pathway and affects the necessary documentation and approval processes for medical devices.
What role do clinical trials play in the regulatory landscape?
Obtaining approval for clinical trials is a fundamental step that involves regional regulatory bodies, and tailored approaches can expedite the approval process, allowing for faster market entry.
What is the significance of quality management systems in the Medtech industry?
Implementing robust quality management systems that comply with ISO 13485 standards is crucial for ensuring product safety and efficacy, enhancing compliance, and building trust with stakeholders.
What are the labeling and advertising requirements for medical devices?
Labels must adhere to regional language requirements and include essential safety information, while advertising practices must align with local regulations to avoid misleading claims.
How is the regulatory landscape evolving, particularly regarding software as a medical device (SaMD)?
The landscape for product classification is evolving, with increased scrutiny on SaMD expected due to likely more stringent requirements from the FDA, particularly concerning cybersecurity risks.
What services does bioaccess™ provide to assist Medtech companies?
Bioaccess™ offers a comprehensive range of clinical trial management services, including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting.
Why is it essential to engage with local experts in navigating regulations?
Engaging with local experts provides valuable insights into the specific nuances that may affect product approval timelines and market strategies, helping companies effectively navigate the regulatory environment.

