Overview
Successfully expanding medtech to Latin America requires a deep understanding of the diverse clinical research landscape, the ability to leverage local expertise, and the skill to navigate regulatory challenges. These elements are crucial for enhancing operational efficiency and patient engagement. This article underscores that strategic collaborations, cost advantages, and tailored approaches to patient recruitment are vital for medtech firms aiming to effectively launch clinical trials and achieve successful outcomes in the region.
Introduction
Latin America is rapidly emerging as a pivotal player in the global clinical research landscape, presenting a rich tapestry of opportunities and challenges. With countries like Brazil, Mexico, and Argentina leading the charge, Colombia—particularly Barranquilla—is becoming a hotspot for clinical trials, driven by strategic collaborations and regulatory advancements. The region's unique demographic diversity not only enhances the quality of research outcomes but also offers a cost-effective alternative to traditional markets.
However, navigating the complex regulatory environments and cultural nuances necessitates a tailored approach. This article delves into the intricacies of conducting clinical trials in Latin America, spotlighting the benefits, challenges, and best practices that can empower Medtech companies to thrive in this dynamic arena.
Understanding the Clinical Research Landscape in Latin America
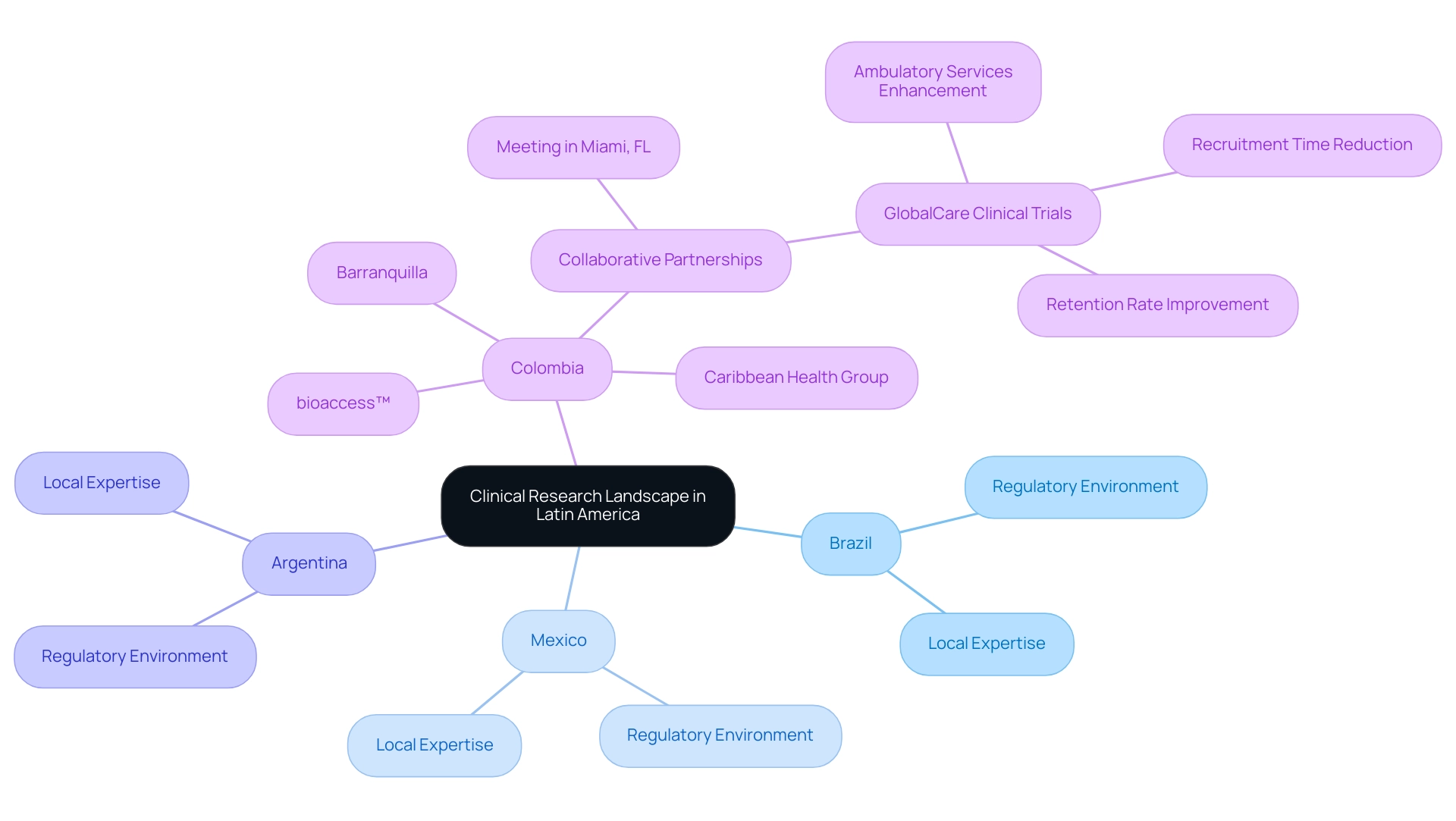
The research landscape in Latin America is characterized by its diversity and rapid evolution, particularly in prominent nations such as Brazil, Mexico, and Argentina, which are crucial for expanding medtech in the region. Notably, Colombia is emerging as a significant player, with Barranquilla being positioned as a leading destination for medical trials through strategic collaborations like that of bioaccess™ and Caribbean Health Group. This partnership was unveiled on March 29, 2019, during a meeting in Miami, FL, supported by Colombia's Minister of Health, who advocates for attracting more research projects to the region.
Moreover, GlobalCare Clinical Trials has joined forces with bioaccess™ to enhance ambulatory services in Colombia, achieving over a 50% reduction in recruitment time and an impressive 95% retention rate. This localized approach is increasingly recognized as a strategic advantage in expanding medtech in Latin America while navigating the complex clinical research landscape. Each nation possesses unique laws and ethics committees that influence timelines and associated costs, making collaboration with regional experts essential.
Such collaborations not only deepen the understanding of these dynamics but also significantly enhance the likelihood of successful execution. The case study titled 'Importance of Local Presence and Leadership in LATAM' further illustrates that expanding medtech in Latin America by leveraging local regulatory expertise has resulted in faster approval timelines and simultaneous launches in key LATAM markets.
Why Latin America is an Attractive Location for Medtech Trials
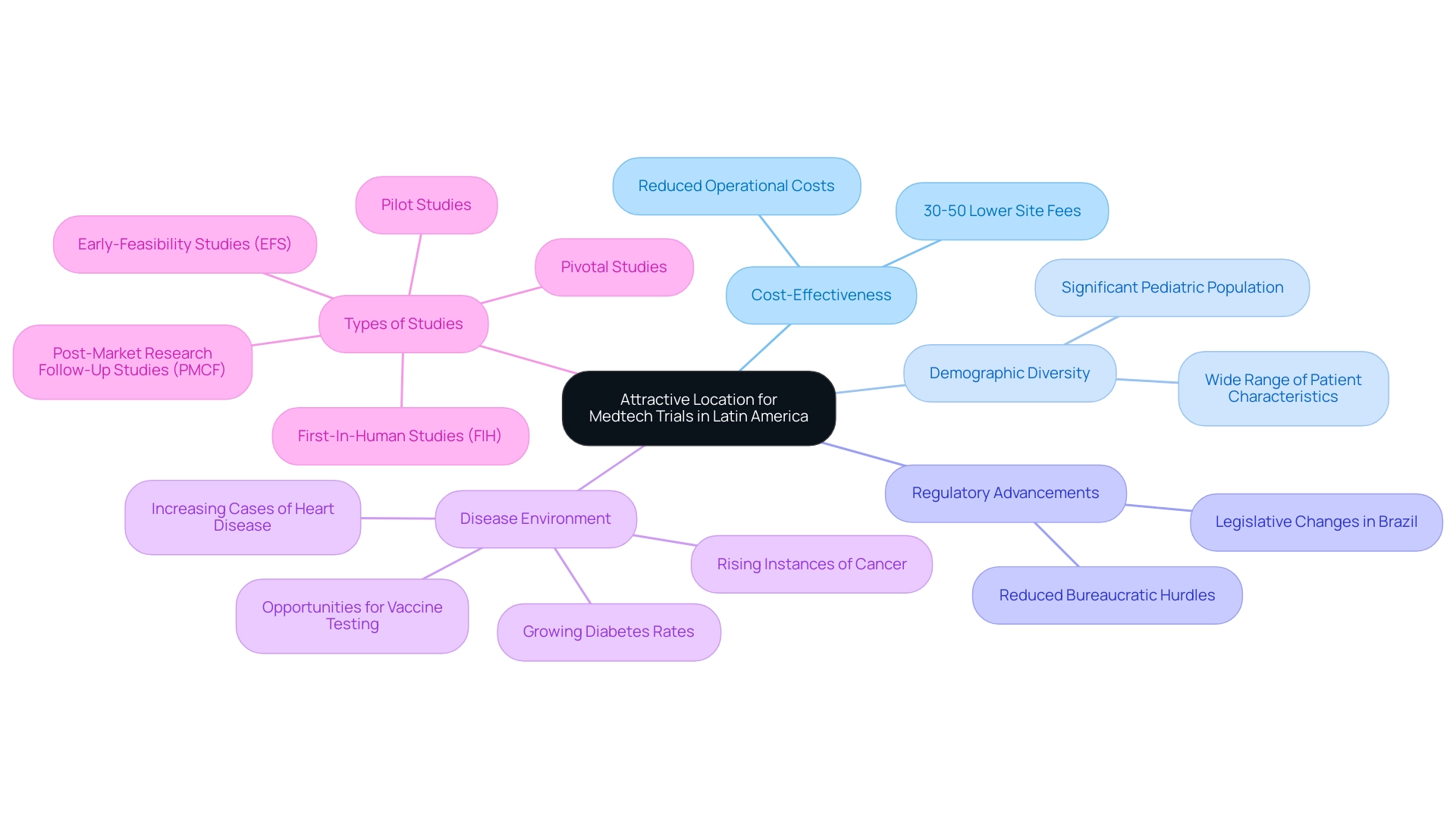
Expanding medtech to Latin America offers a wealth of advantages for clinical studies, particularly in operational cost-effectiveness. With site fees in the U.S. being approximately 30–50% higher than those in regions like Latin America, companies can significantly decrease their expenditures while upholding quality standards. The demographic diversity across Latin American nations serves as another crucial advantage, allowing studies to capture a wide range of patient characteristics that are essential for comprehensive research outcomes.
For example, the region's substantial pediatric population presents significant opportunities for future drug markets. Recent regulatory advancements in Brazil further enhance this landscape; the country has enacted legislative changes designed to reduce bureaucratic hurdles, thus expediting the initiation of medical studies. Additionally, bioaccess® provides accelerated medical device research study services in Latin America, drawing on over 20 years of Medtech expertise in managing various study types, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Research Follow-Up Studies (PMCF)
Our specialized expertise and adaptability empower us to navigate the complexities of research studies with efficiency. As highlighted by Precedence Research, "Factors such as the rising prevalence of chronic disorders, increasing number of studies in developing regions, growing number of biologics, rising demand for advanced treatments such as personalized medicines, outbreak of viral diseases, increasing cases of cancer globally, expanding geriatric population, and growing research and development expenditure are driving the market expansion for studies worldwide." This unique blend of cost savings, demographic diversity, and regulatory support presents a compelling proposition for Medtech firms looking to invest in the expansion of medtech to Latin America.
Moreover, the diverse disease environment, characterized by rising instances of cancer, heart disease, and diabetes, presents numerous opportunities for specialized research, underscoring the critical importance of conducting studies in this region.
Navigating Challenges in Latin American Clinical Research
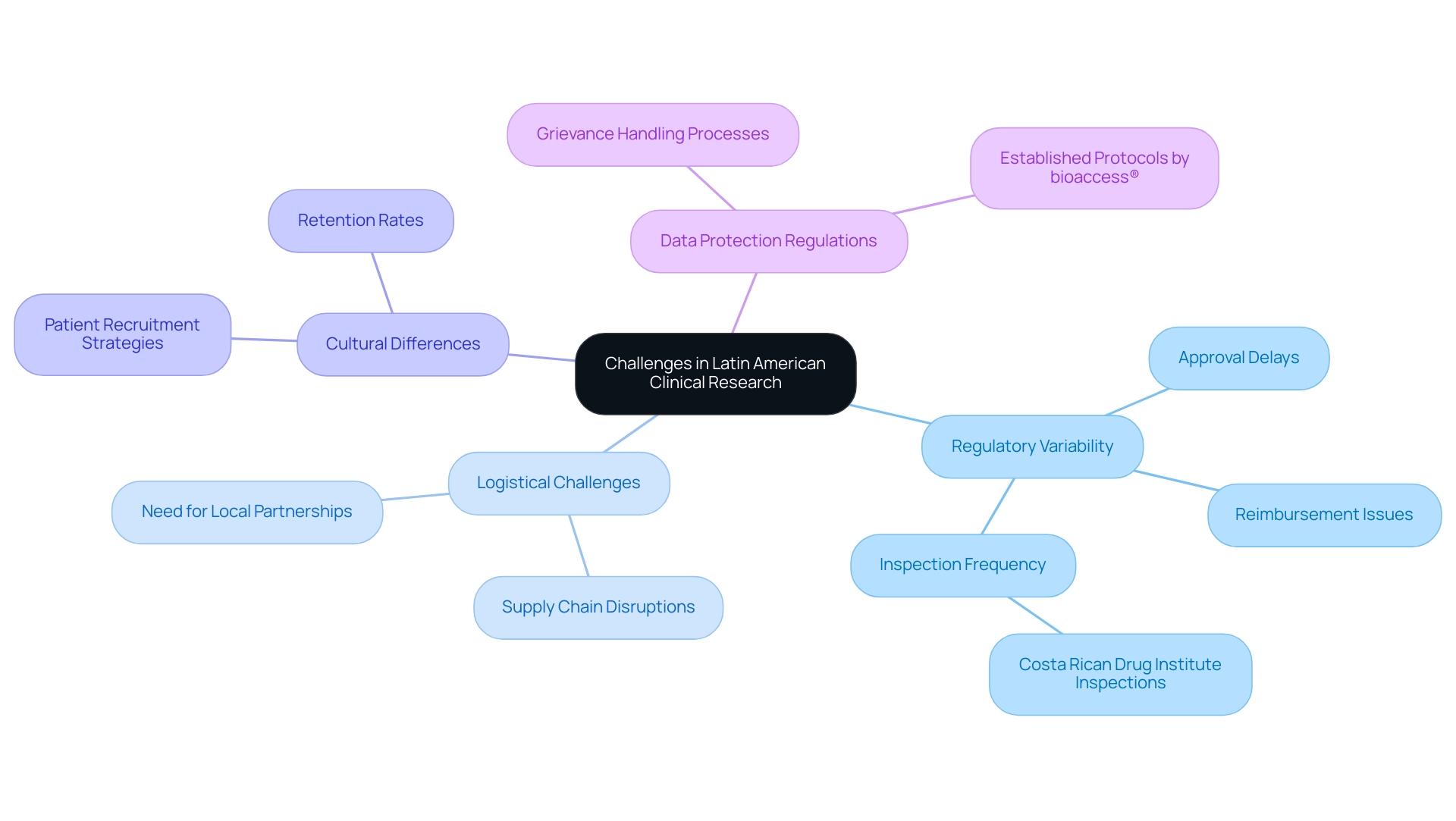
Latin America presents significant advantages for medical research; however, expanding medtech in the region necessitates an awareness of the inherent challenges that accompany these opportunities. A primary concern is the variability in regulatory environments across the region, which can result in substantial delays in approval processes. For instance, a recent study revealed that regulatory approval and reimbursement times for orphan and oncological treatments often exceed five years in countries such as Colombia, Chile, and Mexico, with 87% of cases showing no progress in the past year.
As highlighted by Gotuzzo in 'Clinical Research in Latin America: Constraints and Opportunities,' this situation underscores the urgent need for enhanced dialogue and collaboration among stakeholders. Here, bioaccess® distinguishes itself as a reliable CRO, dedicated to facilitating the expansion of medtech in Latin America by fostering these essential connections and accelerating medical device research. Logistical challenges also play a crucial role; supply chain disruptions and the necessity for local partnerships can significantly complicate trial management.
Moreover, the Costa Rican Drug Institute conducted 26 inspections and one reinspection of companies and/or laboratories in 2024, illustrating the regulatory scrutiny that can impact clinical research timelines. Cultural differences further affect patient recruitment and retention rates, making it imperative for companies to adapt their strategies accordingly. Investing in local expertise, conducting thorough feasibility studies, and nurturing strong relationships with local stakeholders are vital steps to navigate these complexities.
Furthermore, research directors must be cognizant of data protection regulations and grievance handling processes, which bioaccess® addresses through its established protocols. By leveraging bioaccess®'s extensive knowledge of the Latin American medtech landscape and adopting a proactive approach, organizations can effectively mitigate potential setbacks and enhance their research endeavors, crucial for the expansion of medtech in Latin America. For operational guidance, bioaccess® provides user manuals detailing best practices and procedures, ensuring that research directors are well-prepared to manage the complexities of conducting studies in diverse environments.
Embracing Patient-Centric Approaches in Clinical Trials
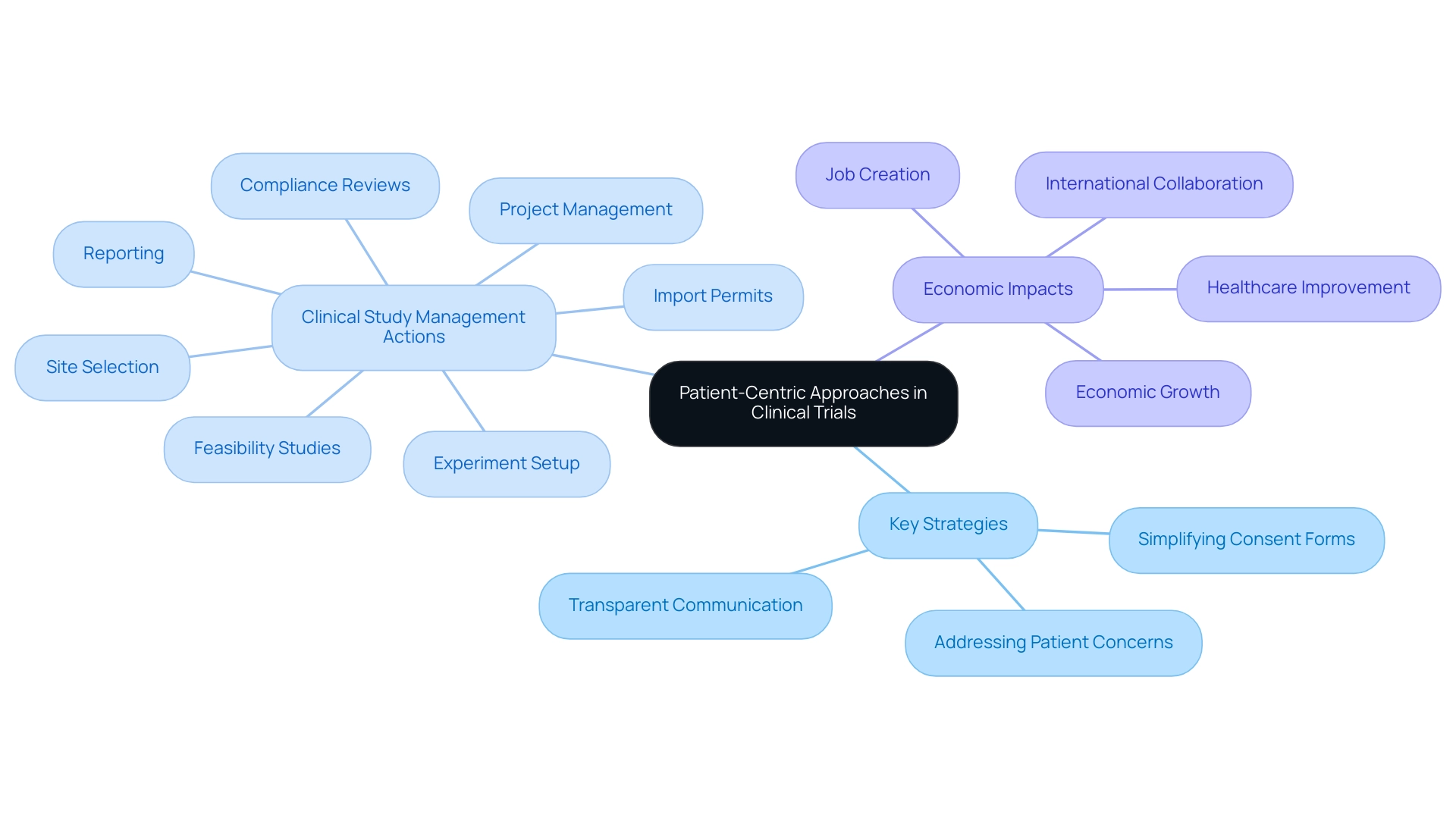
Incorporating patient-centric approaches is vital for the success of clinical studies, particularly in the context of expanding medtech to Latin America. Actively involving individuals throughout the process—from design to execution—ensures that their voices are heard and appreciated. Key strategies for fostering this engagement include:
- Simplifying consent forms
- Providing transparent communication about study details
- Proactively addressing [patient concerns
Dushyanth Surakanti](https://pmc.ncbi.nlm.nih.gov/articles/PMC11186095), Founder & CEO of Sparta Biomedical, shared his insights during bioaccess®'s first human study in Colombia, stating, 'The tailored approaches we implemented were crucial in achieving successful outcomes.' Furthermore, Dr. John B. Simpson's OCT-guided atherectomy research in Cali, Colombia, emphasizes the partnership with LATAM CRO specialists, which is essential for thorough clinical study management. This encompasses:
- Feasibility studies
- Site selection
- Compliance reviews
- Experiment setup
- Import permits
- Project management
- Reporting
bioaccess® specializes in conducting Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), offering the expertise required to manage the intricacies of these evaluations. Furthermore, the impact of Medtech clinical studies on local economies is noteworthy, contributing to:
- Job creation
- Economic growth
- Healthcare improvement
- International collaboration
The insights obtained from participant engagement sessions, such as those carried out in the PPIE session for the PRO-CRM study design, are anticipated to impact future research directions, highlighting the significance of customizing studies to address participant needs.
By leveraging technology, like mobile applications for real-time feedback, Medtech companies can significantly enhance the patient experience, ultimately leading to improved recruitment and retention rates, and more robust and reliable study outcomes.
A Step-by-Step Approach to Launching Clinical Trials in Latin America
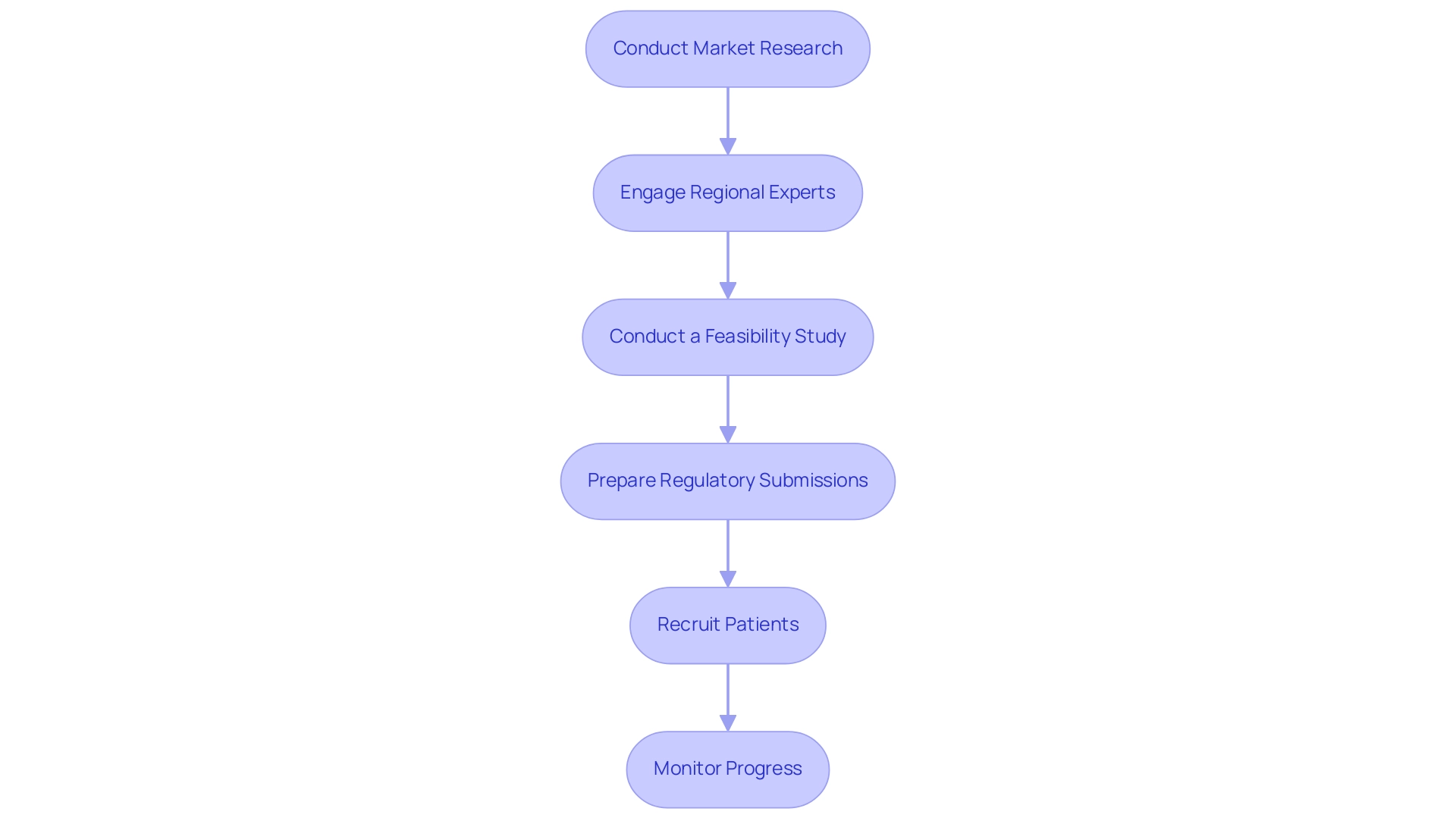
Expanding medtech to Latin America, particularly through launching clinical studies in Colombia, necessitates a structured and informed approach. Here’s a step-by-step guide to navigate this process effectively:
- Conduct Market Research: Begin by understanding local regulations and the unique market dynamics. This insight is critical as it shapes the feasibility and compliance with regional laws. An analysis of 297 medical trials revealed that while most were randomized and blinded, only a small percentage focused on neglected diseases, underscoring the need for targeted market research.
- Engage Regional Experts: Establishing robust connections with nearby research organizations (CROs) and regulatory consultants can provide invaluable insights into the operational landscape. In Colombia, for instance, the presence of high-quality hospitals ranked among the best in Latin America ensures a robust support system for clinical research.
- Conduct a Feasibility Study: Evaluate site capabilities and assess participant availability to ensure that the trial can be successfully executed within the regional context. Colombia's population of over 50 million, with 95% covered by universal healthcare, presents a significant patient recruitment advantage.
- Prepare Regulatory Submissions: Ensure that all regulatory submissions adhere to regional laws. The process in Colombia is notably efficient; the total IRB/EC and MoH (INVIMA) review takes only 90-120 days, which is advantageous for timely trial initiation.
- Recruit Patients: Implement targeted recruitment strategies tailored to resonate with local populations. This approach not only enhances recruitment but also fosters community trust and engagement. Given Colombia's favorable healthcare environment, which includes significant R&D tax incentives such as a 100% tax deduction and a 25% tax discount for investments in science, technology, and innovation projects, your efforts in patient recruitment can be further supported.
- Monitor Progress: Continuously assess performance and adjust strategies as necessary. Regular monitoring can identify potential issues early, allowing for timely interventions. A case study from Brazil exemplifies the importance of personalized approaches. Implementing tailored follow-up communications and educational resources resulted in a remarkable 25% increase in participant retention compared to traditional methods. This statistic emphasizes how understanding community needs can improve study effectiveness and participant involvement.
Henry L. Gómez from Oncosalud-AUNA in Peru highlights, 'In conclusion, the weak financial management of research groups and inadequate regulatory context are barriers found in Latin America; however, medical oncologists perceive the real potential of the region.' This viewpoint underscores the significance of strategic planning and regional insight in addressing challenges and realizing the potential of expanding medtech to Latin America in the field of medical research. Moreover, medical research greatly supports local economies by creating jobs and enhancing healthcare services, emphasizing the essential role that research studies play in promoting economic growth in the area.
Leveraging Technology to Enhance Clinical Trials
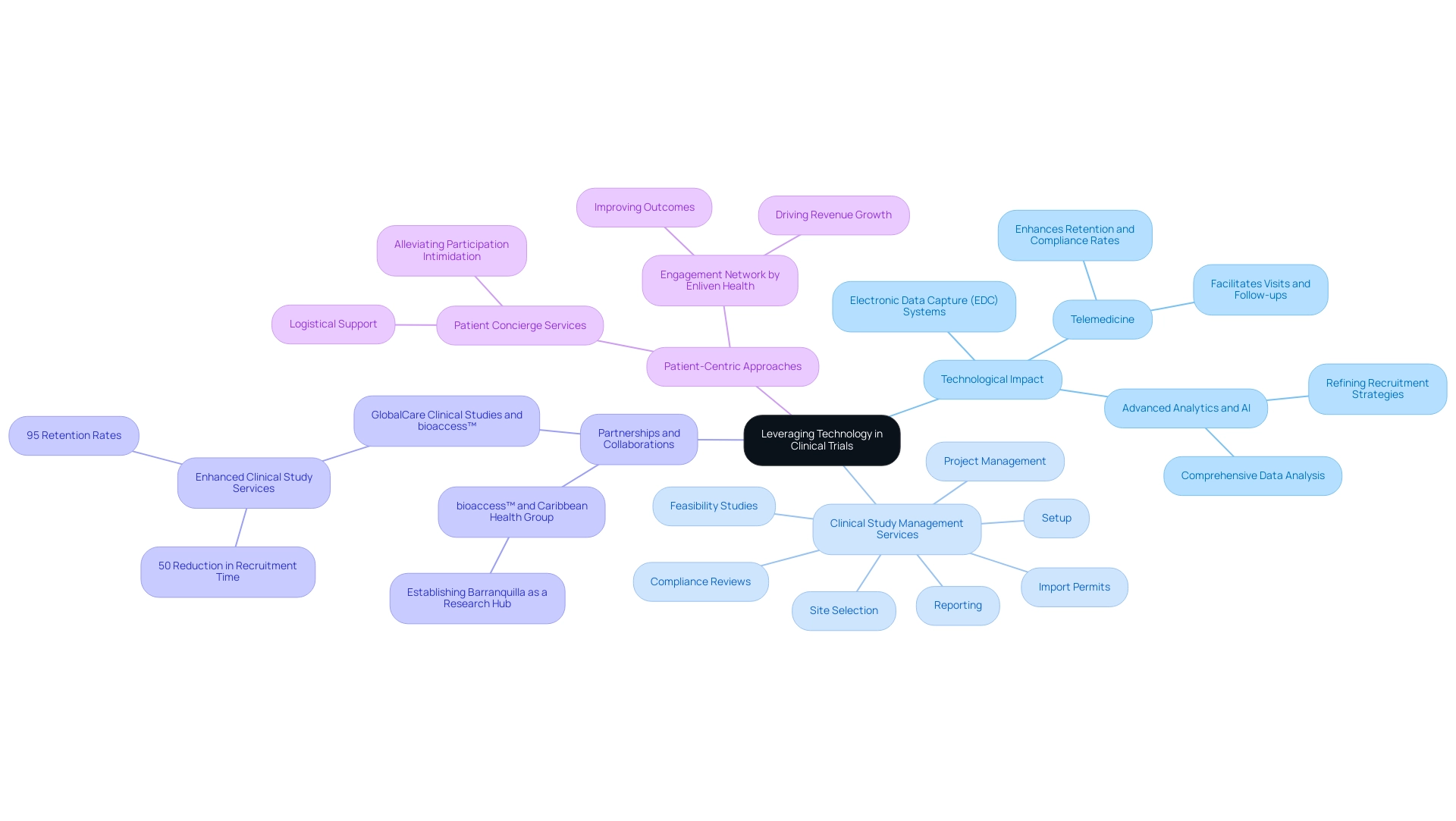
The incorporation of technology is transforming the environment of medical studies, with a significant focus on electronic data capture (EDC) systems that enhance data collection efficiency, minimize errors, and bolster monitoring capabilities. Our comprehensive clinical study management services include:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting
This ensures thorough oversight throughout the research process. Since 2021, the number of marketed digital care programs has nearly doubled, indicating a robust industry-wide shift towards innovative solutions.
Telemedicine, in particular, has emerged as a vital tool, facilitating visits and follow-ups that significantly enhance retention and compliance rates. Scott Gray, CEO of Clincierge, underscores this by stating,
By better understanding a patient’s needs, preferences, and participation barriers, a more efficient and patient-friendly study model can be constructed, ensuring a more positive overall experience.
Moreover, partnerships like that of bioaccess™ with Caribbean Health Group aim to establish Barranquilla as a premier location for medical research in Latin America, supported by Colombia's Minister of Health. This illustrates how the expansion of medtech into Latin America positively influences local economies through job creation and economic development.
Additionally, GlobalCare Clinical Studies' collaboration with bioaccess™ has enhanced clinical study ambulatory services in Colombia, achieving over a 50% reduction in recruitment time and 95% retention rates. Advanced analytics and artificial intelligence are proving essential in refining subject recruitment strategies and conducting comprehensive data analysis, yielding deeper insights into study outcomes. In December 2022, Enliven Health launched the Engagement Network, aimed at improving outcomes and driving revenue growth, aligning with the trend towards consumer-centric approaches.
Furthermore, the case study titled 'Patient Concierge Services at the Heart of Trials' illustrates how sponsors and CROs are implementing these services to tackle recruitment and retention challenges, providing logistical support that alleviates participation intimidation for patients. As Medtech firms adopt these technologies, they not only enhance operational efficiency but also elevate the overall quality of their research studies.
Best Practices for Ensuring Cost Efficiency in Clinical Trials
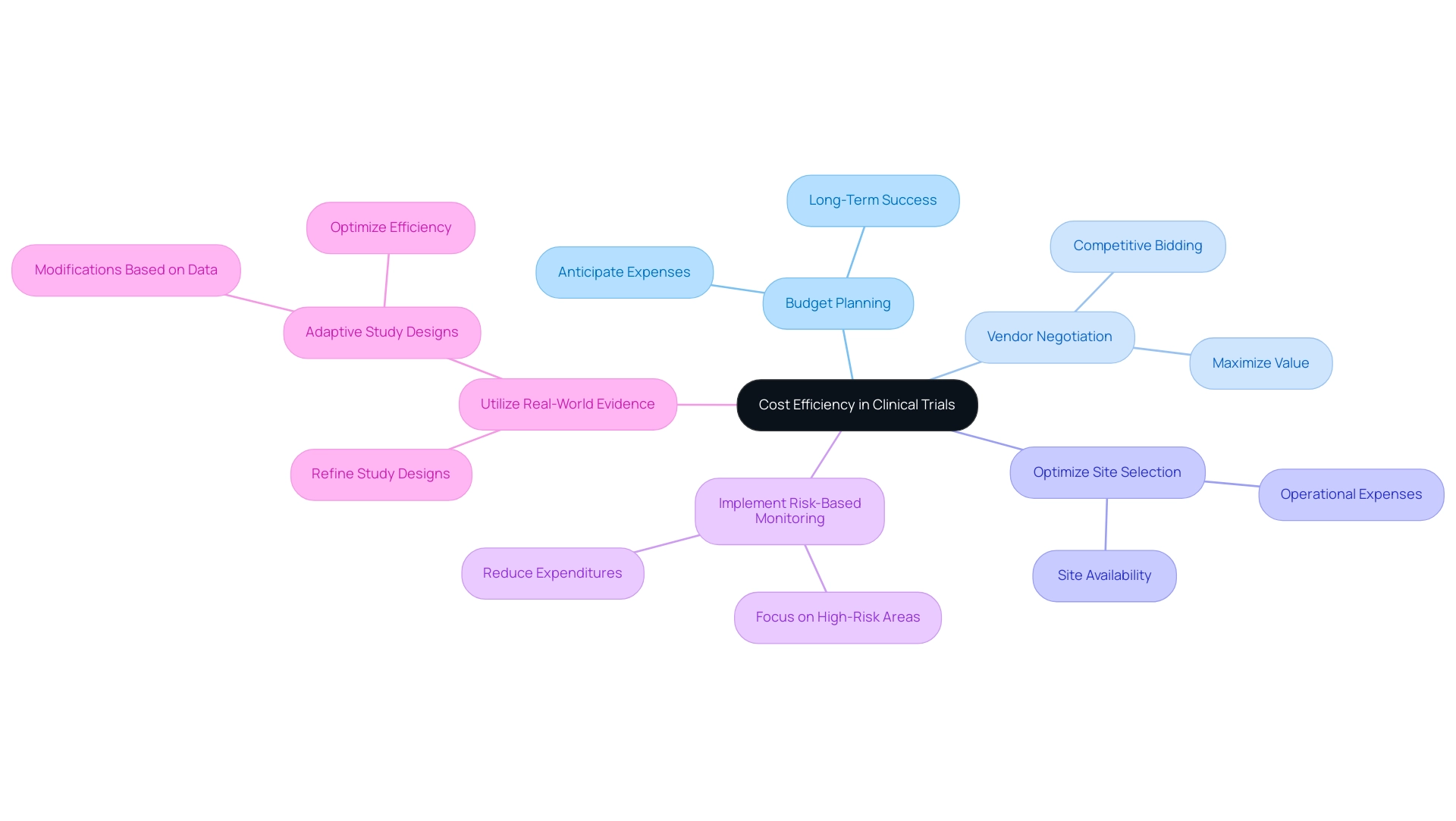
To achieve cost efficiency in research studies, especially as the sector evolves, embracing a comprehensive strategy focused on best practices is essential. With over 20 years of experience in Medtech, bioaccess® specializes in extensive study management services, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF). Our tailored approach ensures that each study is meticulously designed to meet specific client needs and regional challenges.
Consider the following strategies:
- Budget Planning: Establish a thorough budget that anticipates all potential expenses, recognizing that effective budget planning is critical for long-term success. In 2023, data indicated there were 453,803 registered research studies, highlighting the competitive environment where effective budgeting can set successful experiments apart.
- Vendor Negotiation: Engage in competitive bidding processes to negotiate advantageous contracts with vendors and service providers, ensuring maximum value while minimizing costs.
- Optimize Site Selection: Carefully select clinical sites based on availability and operational expenses to enhance efficiency while ensuring robust engagement, a hallmark of bioaccess® in navigating local environments.
- Implement Risk-Based Monitoring: Focus monitoring efforts on high-risk areas, which can significantly reduce unnecessary expenditures and bolster study integrity.
- Utilize Real-World Evidence: Leverage real-world data to refine study designs, potentially reducing costs associated with participant recruitment and enhancing overall efficiency. Moreover, the adoption of adaptive study designs allows for modifications based on accumulating data, optimizing efficiency and potentially shortening timelines.
High patient retention rates not only improve the likelihood of success but also conserve resources and funds allocated to unproductive studies. Additionally, expanding Medtech to Latin America through patient-centric approaches and the integration of concierge services can alleviate recruitment and retention challenges, ensuring that Medtech companies strategically maximize their budgets while preserving the integrity and success of their trials. Ultimately, by partnering with vetted CROs like bioaccess®, U.S. medical device companies can effectively contribute to local economic growth and healthcare enhancement through their clinical studies.
Conclusion
Latin America stands on the brink of becoming a cornerstone of the global clinical research landscape, particularly for Medtech companies in search of innovative and cost-effective solutions. The region's diverse demographics, coupled with recent regulatory advancements, present significant advantages. This is evidenced by the strategic collaborations and successful trials occurring in countries such as Colombia. The case studies highlighted in this article underscore the critical role of local expertise and patient-centric approaches, which not only enhance trial efficiency but also ensure that the needs of diverse populations are met.
Nonetheless, the journey is not devoid of challenges. Navigating the complex regulatory environments and cultural nuances necessitates a tailored strategy. The variability in approval timelines and logistical hurdles underscores the necessity for robust partnerships with local organizations, which can significantly mitigate risks and streamline the trial process. By investing in local knowledge and fostering strong stakeholder relationships, companies can effectively surmount these barriers.
Ultimately, as Medtech firms leverage the opportunities presented by Latin America, they contribute not only to their own success but also to driving economic growth and healthcare advancements within the region. The integration of technology and patient engagement strategies further enhances the potential for successful clinical trials, paving the way for innovative treatments that address the unique needs of Latin American populations. This dynamic landscape is ripe for exploration, and with the right approach, Medtech companies can thrive in this burgeoning market.
Frequently Asked Questions
What is the current research landscape in Latin America regarding medtech?
The research landscape in Latin America is diverse and rapidly evolving, particularly in key countries like Brazil, Mexico, and Argentina. Colombia is emerging as a significant player, with Barranquilla becoming a leading destination for medical trials through partnerships like that of bioaccess™ and Caribbean Health Group.
How has Colombia positioned itself in the medtech research field?
Colombia is positioning itself as a key player in medtech research, with Barranquilla being highlighted as a leading destination for medical trials. This positioning is supported by strategic collaborations, such as the one between bioaccess™ and Caribbean Health Group, which aims to attract more research projects to the region.
What advantages do local collaborations provide in expanding medtech in Latin America?
Local collaborations enhance the understanding of regional dynamics and significantly improve the chances of successful execution of clinical trials. They also help navigate unique laws and ethics committees in each country, leading to faster approval timelines and simultaneous launches in key LATAM markets.
What operational cost advantages does Latin America offer for clinical studies?
Latin America offers significant operational cost advantages, with site fees approximately 30–50% lower than in the U.S. This allows companies to decrease expenditures while maintaining quality standards in their clinical studies.
How does demographic diversity in Latin America benefit clinical research?
The demographic diversity across Latin American nations allows studies to capture a wide range of patient characteristics, which is essential for comprehensive research outcomes. The region's substantial pediatric population also presents significant opportunities for future drug markets.
What recent regulatory changes in Brazil support medtech expansion?
Brazil has enacted legislative changes designed to reduce bureaucratic hurdles, expediting the initiation of medical studies, which enhances the research landscape for medtech firms.
What types of studies does bioaccess® specialize in for medtech research in Latin America?
bioaccess® specializes in various study types, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Research Follow-Up Studies (PMCF).
What factors are driving the expansion of clinical studies in Latin America?
Factors driving the expansion include the rising prevalence of chronic disorders, increasing studies in developing regions, growing demand for advanced treatments, outbreaks of viral diseases, rising cancer cases, an expanding geriatric population, and increasing research and development expenditure.
What opportunities does the diverse disease environment in Latin America present?
The diverse disease environment, characterized by rising instances of cancer, heart disease, and diabetes, presents numerous opportunities for specialized research, highlighting the importance of conducting studies in this region.




