Overview
This article serves as a crucial guide for navigating the regulatory requirements for medtech trials in Ecuador. It emphasizes the framework established by Ministerial Agreement 0075-2017 and highlights the pivotal role of key regulatory authorities such as ARCSA. Understanding local regulations, ethical considerations, and logistical challenges is essential for ensuring compliance and the successful execution of clinical research. By addressing these factors, Ecuador positions itself as a competitive destination for medtech studies, appealing to stakeholders in the clinical research landscape.
In the evolving medtech environment, Bioaccess plays a significant role in tackling these challenges. The complexities of regulatory compliance can be daunting, yet they are critical for fostering a robust clinical research ecosystem. By collaborating with local authorities and understanding the nuances of the regulatory landscape, researchers can effectively navigate these hurdles.
In conclusion, the importance of collaboration among stakeholders cannot be overstated. As Ecuador continues to develop its medtech capabilities, embracing these regulatory frameworks will be key to attracting international research initiatives. The next steps involve engaging with local experts and regulatory bodies to ensure a seamless process for clinical trials.
Introduction
Ecuador is emerging as a noteworthy hub for Medtech trials, driven by its evolving regulatory framework aimed at ensuring safety and efficacy in clinical research. The Ministerial Agreement 0075-2017 establishes essential guidelines, and recent updates have further enhanced these regulations. This positions the country as a competitive alternative for companies grappling with challenges in more complex markets like the U.S.
As organizations strive to navigate the intricacies of regulatory compliance, grasping the roles of key authorities and the step-by-step approval process becomes crucial. This article explores the regulatory landscape of Medtech trials in Ecuador, underscoring the opportunities and challenges that lie ahead for stakeholders in this burgeoning field.
Overview of Regulatory Framework for Medtech Trials in Ecuador
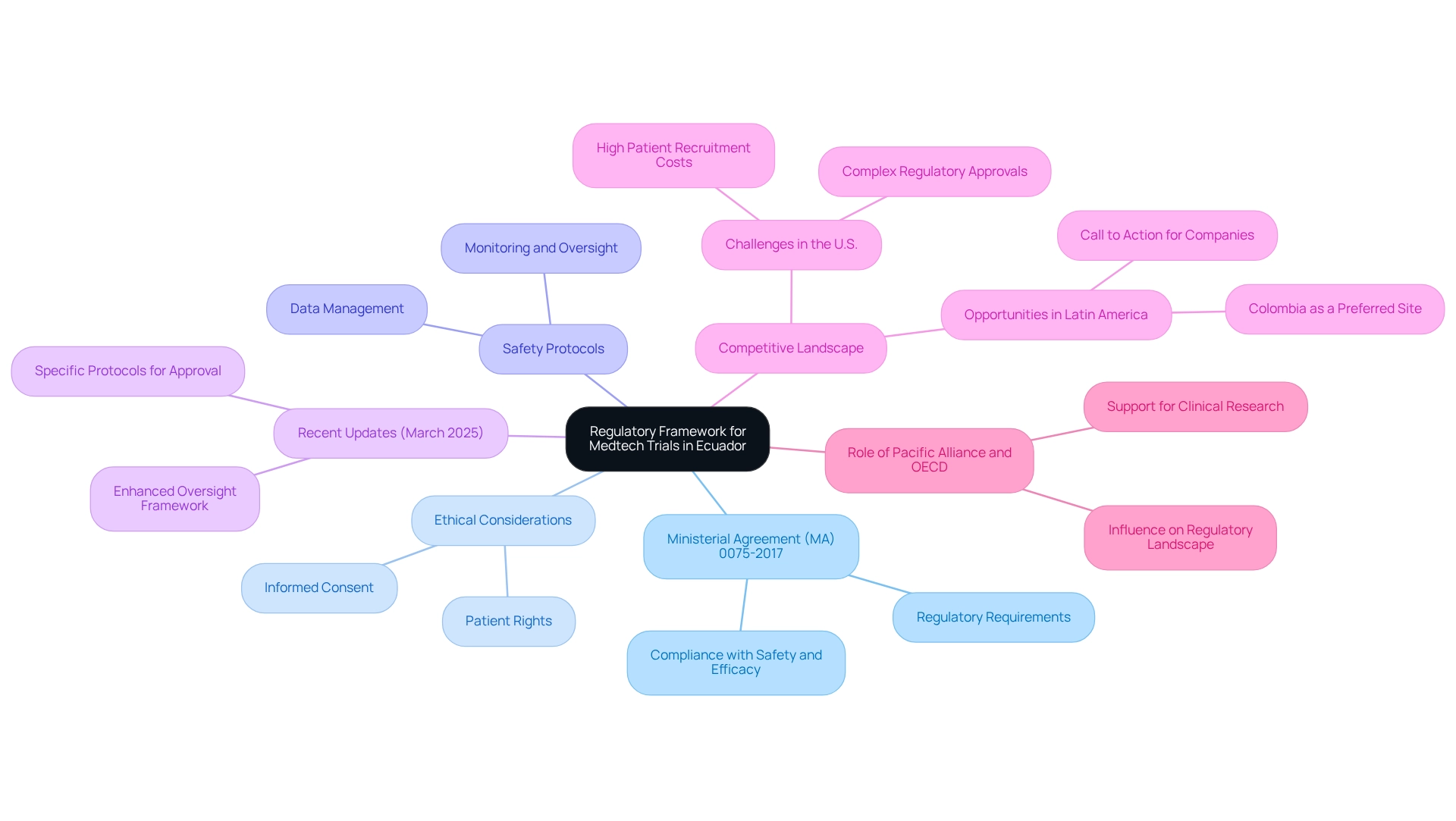
The framework governing medtech studies in the country is predominantly shaped by Ministerial Agreement (MA) 0075-2017, which establishes the regulatory requirements for medtech trials in Ecuador. This framework addresses vital aspects such as ethical considerations, safety protocols, and data management, ensuring compliance with regulatory requirements for medtech trials in Ecuador to guarantee the safety and efficacy of medical devices. Recent updates, introduced in March 2025, have further refined the regulatory requirements for medtech trials in Ecuador by outlining specific protocols for the approval, authorization, execution, oversight, and control of clinical studies. These enhancements reflect a commitment to improving the oversight framework, positioning the country as a more attractive destination for Medtech studies.
In the Latin American context, the regulatory framework of this country is particularly pertinent as companies encounter substantial challenges in the U.S., including high costs for patient recruitment and complex regulatory approvals. Colombia, for instance, is emerging as a preferred site for research studies due to these challenges, underscoring the competitive advantages of conducting studies in that nation. Industry leaders have issued a call to action, stressing the necessity for companies to explore opportunities in Latin America, thereby reinforcing the importance of understanding local regulations for successful market entry.
Moreover, the hurdles faced by U.S. firms in clinical trials underscore the practical implications of the regulatory requirements for medtech trials in Ecuador. A recent case study illustrates that patient recruitment in the U.S. is often costly and time-consuming, prompting companies to seek faster approval processes and cost efficiencies abroad.
Julio Martinez-Clark, CEO and co-founder of bioaccess, highlights the significance of the Pacific Alliance and OECD in shaping the regulatory landscape, stating, "Pay attention to the Pacific Alliance, pay attention to the OECD." With bioaccess's expertise in comprehensive clinical study management services—including feasibility studies, site selection, compliance reviews, study setup, import permits, project management, and reporting—grasping these regulations is essential for compliance and the successful execution of clinical research in the country. This is particularly important as the demand for efficient and effective testing procedures continues to rise in the region, positioning it as a competitive choice for Medtech evaluations compared to other nations.
Key Regulatory Authorities and Their Roles in Medtech Trials
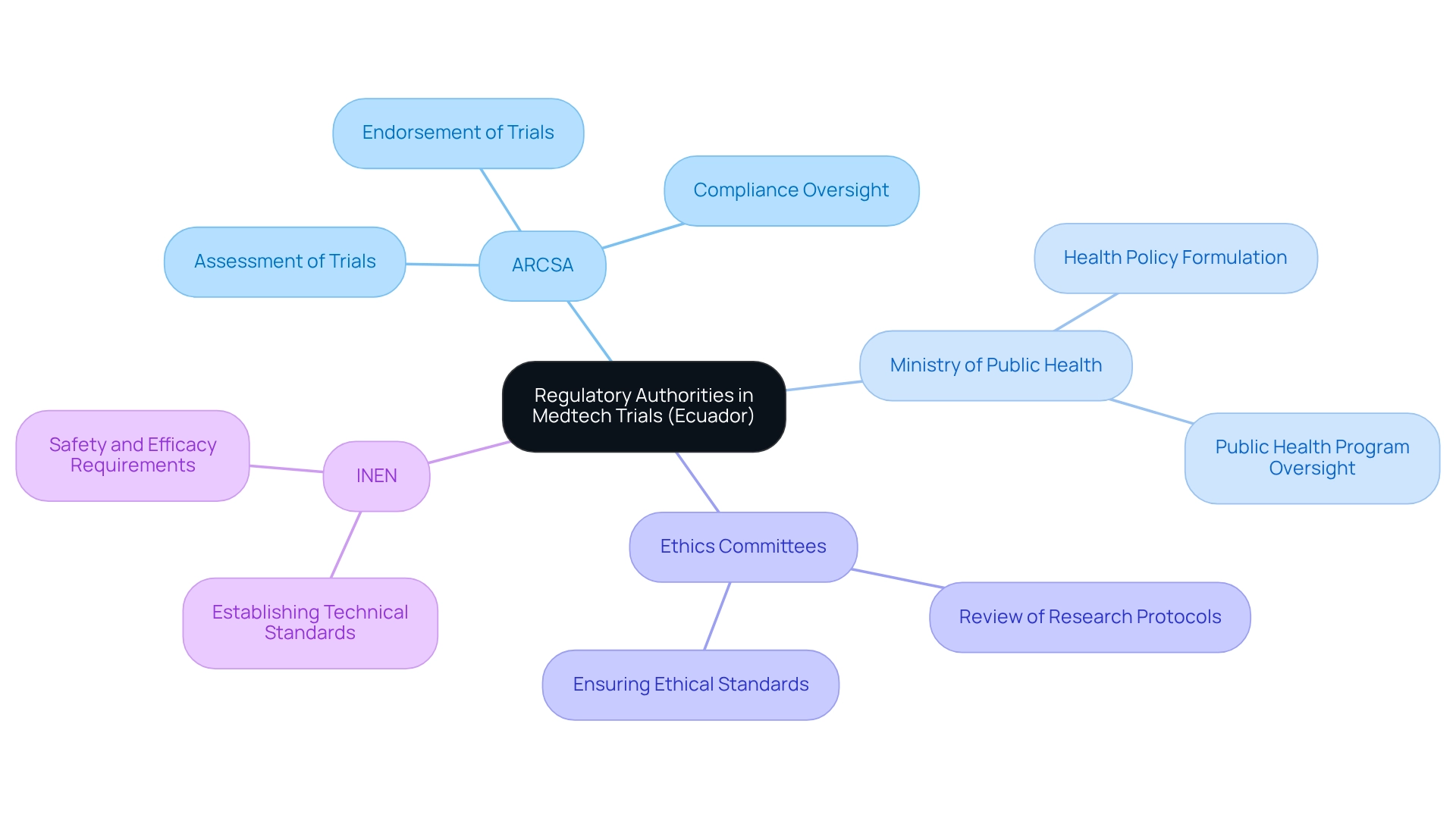
In Ecuador, the National Agency for Regulation, Control, and Sanitary Surveillance (ARCSA) serves as the primary regulatory authority that oversees the regulatory requirements for medtech trials in Ecuador. ARCSA's responsibilities include the assessment and endorsement of trials, ensuring strict compliance with the regulatory requirements for medtech trials in Ecuador. This agency plays a critical role in upholding the integrity and safety of clinical research, particularly concerning the regulatory requirements for medtech trials in Ecuador, as the Medtech sector undergoes continuous evolution.
The Ministry of Public Health bolsters ARCSA's efforts by formulating health policies and overseeing public health programs, which are vital components of the governance framework. Additionally, ethics committees are integral, meticulously reviewing research protocols to guarantee that ethical standards are upheld throughout the research process. The Ecuadorian Institute of Standardization (INEN) further contributes by establishing technical standards for medical devices, ensuring they adhere to safety and efficacy requirements.
Engaging these oversight organizations early in the research process is essential for effective study management of the regulatory requirements for medtech trials in Ecuador. bioaccess® provides comprehensive clinical trial management services, including feasibility studies, compliance reviews, trial setup, import permits, project management, and reporting. This expertise is crucial for navigating the complexities of compliance in Ecuador, especially concerning the regulatory requirements for medtech trials in Ecuador, which medical device startups encounter amid challenges such as legal obstacles and competition.
For example, insights from the case study titled 'Opportunities in Regulatory Systems Against Counterfeits' emphasize the necessity of regulatory requirements for medtech trials in Ecuador to thwart the manufacturing and commercialization of counterfeit medical devices, highlighting ARCSA's vital role in ensuring compliance and safety.
Moreover, a citation from WCO IPM underscores the importance of communication: "Very interactive and provided a platform for information sharing," which enhances the collaborative aspect of managing compliance demands among Medtech companies and regulatory bodies.
As the landscape of clinical studies in Ecuador continues to evolve, it is imperative for Medtech companies to understand the regulatory requirements for medtech trials in Ecuador to navigate the complexities of clinical research effectively. With over 20 years of experience in the Pharmaceutical, Medical Devices, and Biotechnology sectors, experts like José Camara Santiago offer invaluable insights into the regulatory compliance landscape, further solidifying the significance of these regulatory frameworks.
Step-by-Step Guide to Navigating the Regulatory Approval Process
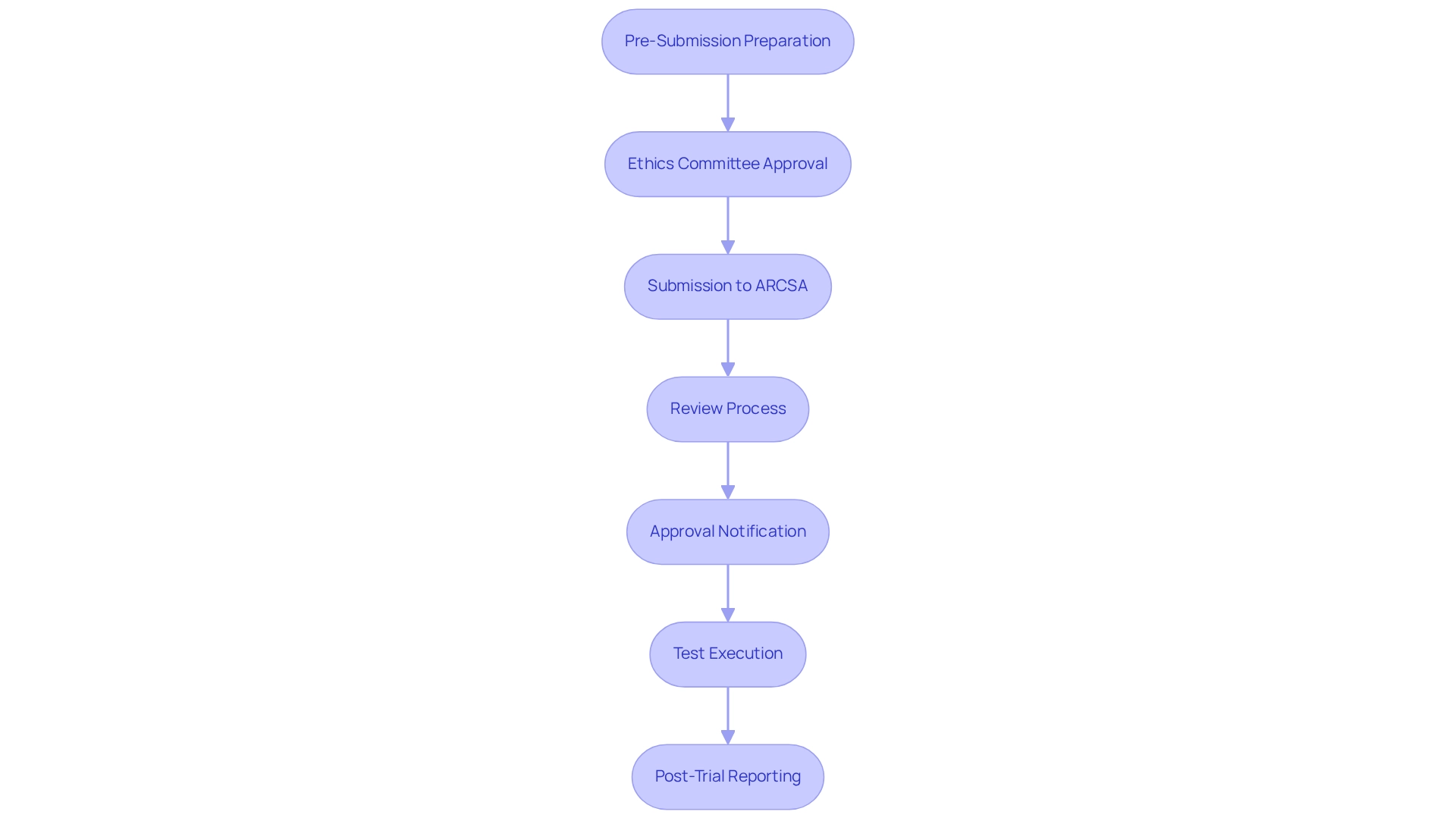
- Pre-Submission Preparation: Engage in discussions with ARCSA to clarify requirements and compile the necessary documentation. This includes creating a comprehensive registration dossier that outlines the study's objectives, methodology, and ethical considerations. Given that the average duration for ARCSA approval of clinical studies is approximately 90 days, meticulous pre-submission preparation is crucial for ensuring a seamless process. Utilizing the expertise of bioaccess® can significantly enhance this preparation phase, as they specialize in navigating the regulatory requirements for medtech trials in Ecuador.
- Ethics Committee Approval: Submit the research protocol to an ethics committee for review. This step is vital to ensure that the study adheres to ethical standards and protects participant rights.
- Submission to ARCSA: Following ethics approval, submit the trial application to ARCSA, including all required documentation such as the ethics committee's approval letter. Bioaccess® can assist in ensuring that all documentation complies with the necessary standards.
- Review Process: ARCSA will conduct a thorough review of the application, which may involve additional queries or requests for clarification. Be prepared to respond promptly to facilitate the review process, leveraging bioaccess®'s expertise in managing communications with oversight authorities.
- Approval Notification: Upon successful review, ARCSA will issue an approval certificate, permitting the experiment to commence. Ensure that all stakeholders are informed of this approval related to the regulatory requirements for medtech trials in Ecuador, and consider bioaccess®'s project management services to streamline this communication.
- Test Execution: Execute the test according to the approved protocol, ensuring compliance with all regulatory requirements throughout the study. Bioaccess® provides extensive clinical study management services, including setup and compliance evaluations, to effectively support the regulatory requirements for medtech trials in Ecuador.
- Post-Trial Reporting: After the trial concludes, submit a final report to ARCSA detailing the outcomes and any adverse events that occurred during the study. This report is essential for meeting the regulatory requirements for medtech trials in Ecuador and informs future research endeavors. Bioaccess® can assist in gathering and presenting this report to ensure it meets all compliance expectations.
In Ecuador, the oversight environment is evolving, particularly concerning the expanding biologics market, which is projected to grow at a compound annual growth rate of 15% until 2027. This growth introduces unique challenges, especially with the new generation of monoclonal antibodies (mAbs), necessitating innovative strategies to manage the intricacies of Medtech evaluations. The demand for specialized services capable of effectively addressing these challenges is underscored by case studies such as the "Monoclonal Antibodies Market Expansion," which highlights the importance of a one-stop shop approach in this rapidly evolving market. By understanding these dynamics, stakeholders can better prepare for the approval process and ensure successful outcomes.
Challenges and Considerations for Conducting Medtech Trials in Ecuador
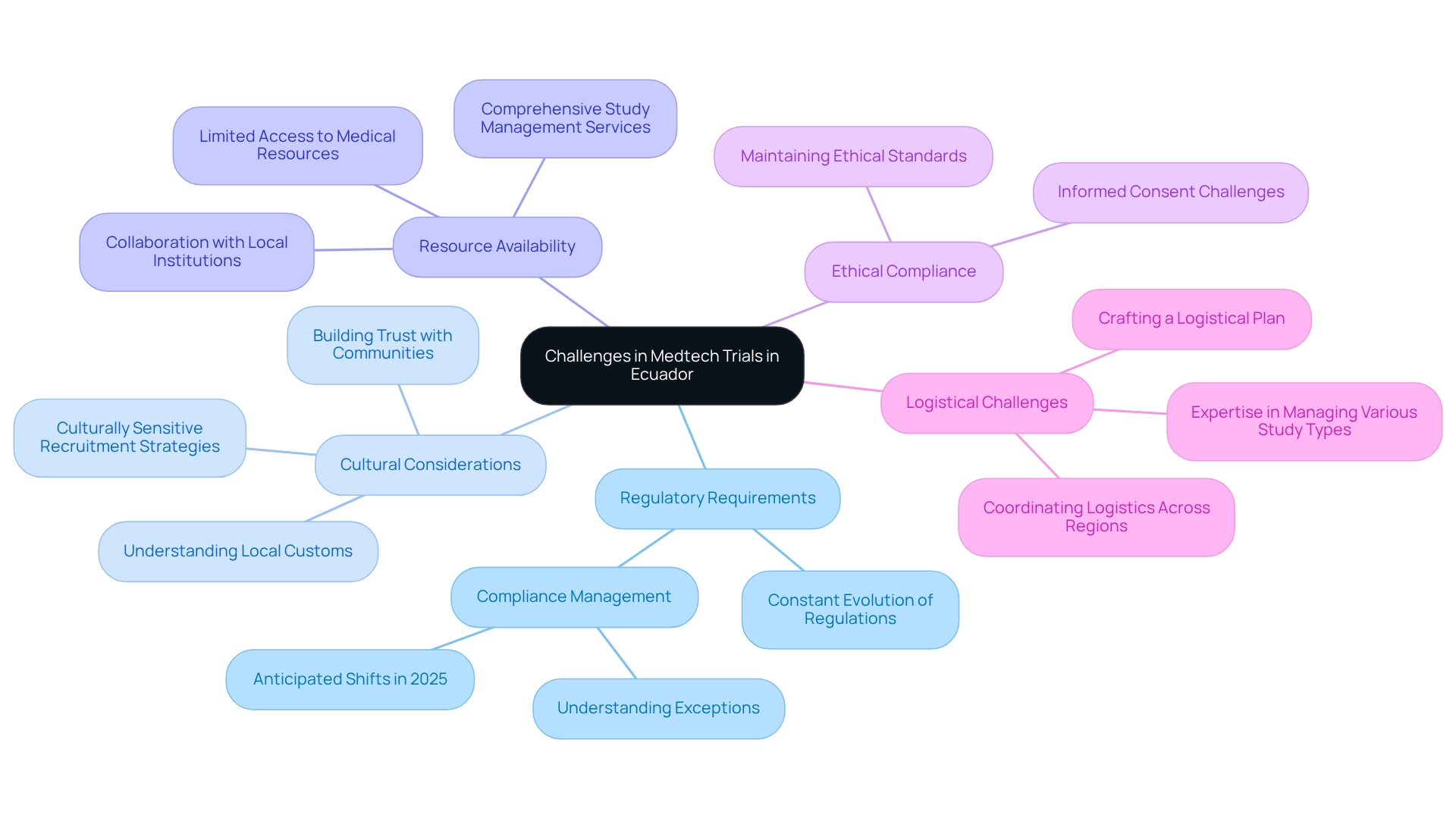
Conducting Medtech trials in Ecuador presents a variety of challenges that can significantly influence the success of clinical research initiatives, largely due to the regulatory requirements for medtech trials in Ecuador, which are in a state of constant evolution and introduce additional layers of complexity. As Dr. Mike Drues, a compliance consultant, aptly states, 'Compliance professionals understand the rules; the finest ones are aware of the exceptions.' Staying abreast of these changes is essential for adherence and effective management, particularly concerning the regulatory requirements for medtech trials in Ecuador, especially with the anticipated shifts in 2025. With over 20 years of experience in Medtech, partnering with bioaccess® equips stakeholders with the necessary expertise to navigate these complexities, ensuring that all regulatory requirements for medtech trials in Ecuador are efficiently met.
Cultural Considerations: A profound understanding of local customs and practices is crucial for effective participant recruitment and retention. Case studies have consistently shown that fostering connections with local communities builds trust and enhances collaboration, which is vital for success in legal proceedings. Recognizing cultural nuances can dramatically influence recruitment strategies and participant engagement, and bioaccess®'s extensive experience in the region is instrumental in developing culturally sensitive approaches.
Resource Availability: Access to specific medical resources and technologies can be limited, potentially obstructing the execution of the study. Insights from Deloitte underscore the significance of human capital management in the Medtech sector, highlighting that collaboration with local institutions can alleviate these challenges by providing essential support and resources. bioaccess® emphasizes comprehensive study management services, including site selection and project oversight, to optimize resource utilization.
Ethical Compliance: Upholding ethical standards is paramount in clinical research. Researchers must guarantee that all participants are fully informed and provide consent, which can be particularly daunting in diverse populations with varying levels of health literacy. Addressing these ethical considerations is vital for maintaining the integrity of the experiment, and bioaccess® is committed to ensuring ethical adherence throughout the study process.
Logistical Challenges: Coordinating logistics for experimental materials and personnel across various regions can be intricate. A meticulously crafted logistical plan is essential to ensure smooth operations and timely execution of the trial. bioaccess®'s expertise in managing logistics for different types of studies—including Early-Feasibility, First-In-Human, and Post-Market Follow-Up Studies—can significantly enhance operational efficiency.
By proactively addressing these challenges and implementing effective strategies, stakeholders can markedly improve the likelihood of successful trial outcomes in Ecuador, leveraging the specialized services offered by bioaccess®.
Conclusion
Ecuador's emergence as a competitive hub for Medtech trials is underscored by its robust regulatory framework, primarily shaped by the Ministerial Agreement 0075-2017. Recent updates have enhanced these regulations, positioning the country as a viable alternative for companies facing challenges in more intricate markets like the U.S. Understanding the roles of key regulatory authorities, such as ARCSA and the Ministry of Public Health, enables stakeholders to navigate the complexities of clinical trials more effectively.
The step-by-step guide to the regulatory approval process emphasizes the importance of thorough preparation, ethics committee engagement, and effective communication with regulatory bodies. With an average approval time of around 90 days, meticulous planning and expert guidance are paramount, particularly as the Medtech sector in Ecuador continues to grow. This growth is further highlighted by the expanding biologics market, which presents unique challenges and opportunities for innovation.
However, conducting Medtech trials in Ecuador is not without its challenges. Regulatory complexity, cultural considerations, and resource availability require careful navigation. By proactively addressing these challenges and leveraging the expertise of organizations like bioaccess, stakeholders can enhance their chances of success. As Ecuador's regulatory landscape continues to evolve, the potential for fruitful Medtech trials remains promising, offering a landscape rich with opportunities for innovation and collaboration in the field of clinical research.
Frequently Asked Questions
What is the primary framework governing medtech studies in Ecuador?
The primary framework is Ministerial Agreement (MA) 0075-2017, which establishes the regulatory requirements for medtech trials in the country.
What aspects does the regulatory framework for medtech trials in Ecuador address?
The framework addresses ethical considerations, safety protocols, and data management to ensure compliance with regulatory requirements and guarantee the safety and efficacy of medical devices.
What recent updates were made to the regulatory requirements for medtech trials in Ecuador?
In March 2025, updates were introduced that refined the regulatory requirements by outlining specific protocols for the approval, authorization, execution, oversight, and control of clinical studies.
Why are these updates significant for medtech studies in Ecuador?
These enhancements improve the oversight framework and position Ecuador as a more attractive destination for medtech studies, especially in comparison to other countries facing regulatory challenges.
What challenges do companies face in the U.S. regarding clinical trials?
Companies encounter high costs for patient recruitment and complex regulatory approvals, making it difficult to conduct clinical trials efficiently.
How does the regulatory environment in Ecuador compare to that of Colombia for research studies?
Colombia is emerging as a preferred site for research studies due to the challenges faced in the U.S., highlighting the competitive advantages of conducting studies in Latin America, including Ecuador.
What role does Julio Martinez-Clark emphasize regarding the Pacific Alliance and OECD?
He stresses the importance of these organizations in shaping the regulatory landscape for medtech studies, advising companies to pay attention to their influence.
What services does bioaccess provide to assist with compliance in clinical studies?
Bioaccess offers comprehensive clinical study management services, including feasibility studies, site selection, compliance reviews, study setup, import permits, project management, and reporting.
Why is understanding local regulations essential for companies looking to enter the Ecuadorian market?
Grasping these regulations is crucial for compliance and the successful execution of clinical research, especially as demand for efficient testing procedures rises in the region.




