Overview
The article addresses best practices and strategies for optimizing medical device clinical trials, underscoring the critical nature of robust study design, regulatory compliance, and effective data management. It asserts that the implementation of:
- Clear objectives
- Active stakeholder engagement
- The utilization of digital tools
can markedly enhance trial efficiency and success rates. Ultimately, these strategies ensure that innovative medical devices are safely and effectively introduced to the market, reinforcing the necessity of a well-structured approach in clinical research.
Introduction
In the rapidly evolving landscape of medical technology, clinical trials serve as the backbone for ensuring the safety and efficacy of innovative devices before they reach the market. These trials, essential for regulatory approval, encompass a variety of methodologies, each tailored to address specific stages of device development.
From pilot studies that lay the groundwork for feasibility to pivotal studies that provide definitive evidence for regulatory compliance, understanding the nuances of these trials is crucial for stakeholders. As the demand for effective clinical research grows—particularly in emerging markets like Latin America—the emphasis on robust trial design and operational efficiency becomes paramount.
This article delves into the types of medical device clinical trials, the regulatory challenges they face, and the best practices that can enhance their success, ultimately paving the way for groundbreaking advancements in patient care.
Understanding Medical Device Clinical Trials: Types and Importance
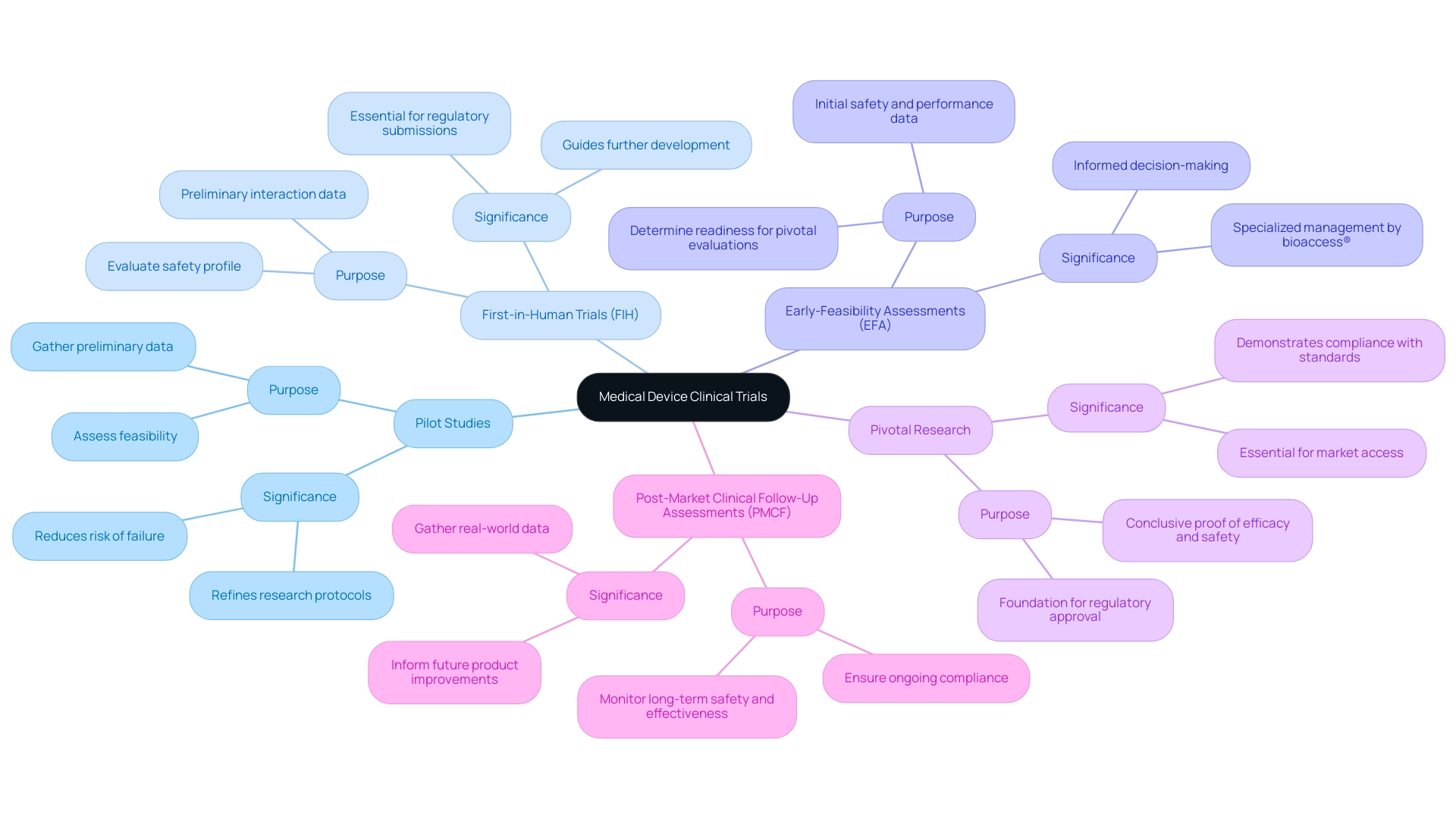
Clinical studies of medical devices are pivotal in evaluating the safety and efficacy of innovative technologies prior to their market introduction. These trials are categorized into several key types, each serving a distinct purpose in the development process:
-
Pilot Studies: These early-stage trials are essential for assessing feasibility and gathering preliminary data. They assist in recognizing potential challenges and refining research protocols, ultimately increasing the likelihood of success in subsequent phases. Statistics indicate that pilot experiments can significantly reduce the risk of failure in later stages by providing valuable insights early in the development process.
-
First-in-Human Trials (FIH): As the inaugural experiments involving human subjects, FIH trials are crucial for evaluating the safety profile of new medical devices. They provide preliminary information on how the device interacts with the human body, which is essential for guiding further development and regulatory submissions.
-
Early-Feasibility Assessments (EFA): These assessments focus on gathering initial safety and performance information from a small group of patients. EFAs are vital in determining whether a device is prepared for larger-scale pivotal evaluations, enabling researchers to make informed decisions about the device's viability. bioaccess® specializes in managing EFAs, leveraging over 20 years of Medtech expertise and a tailored approach to ensure successful outcomes.
-
Pivotal Research: Designed to provide conclusive proof of a device's efficacy and safety, pivotal research typically involves large-scale trials that serve as the foundation for regulatory approval. The results of these investigations are essential for demonstrating compliance with regulatory standards and gaining market access.
-
Post-Market Clinical Follow-Up Assessments (PMCF): Conducted after a device has been introduced to the market, PMCF assessments monitor long-term safety and effectiveness. These studies are crucial for ensuring ongoing compliance with regulatory requirements and for gathering real-world data that can inform future product improvements.
Understanding these various types of medical studies is essential for stakeholders in the healthcare technology sector, particularly in the context of optimizing medical device clinical trials. As the landscape of medical studies evolves, especially in regions like Latin America where the expansion of medtech studies is significant, optimizing medical device clinical trials through robust study design is of utmost importance. Recent trends suggest a shift towards insourcing information management to enhance study quality and control, reflecting a broader industry movement towards operational efficiency and improved patient outcomes.
For instance, 45% of Alcon's information is entered on the same day as the visit date, underscoring the importance of timely information management in clinical trials. As noted by the Head of Clinical Information Engineering, "Traditionally, information management was outsourced to our CRO vendor partners." Part of the initiative is to bring all our research in-house so that our internal teams can start working on it.
This approach allows for greater involvement, enabling us to manage our information effectively and provide high-quality care for our patients. By utilizing insights from preliminary investigations and FIH experiments, stakeholders can navigate the complexities of research more effectively, ensuring that innovative medical devices reach the market safely and efficiently. Furthermore, the focus on MDR solutions is vital for optimizing medical device clinical trials, which encompasses enhancing study design, data collection, analysis, and submission, aligning with ongoing efforts to optimize research in this evolving environment.
Navigating Regulatory Challenges in Medical Device Trials
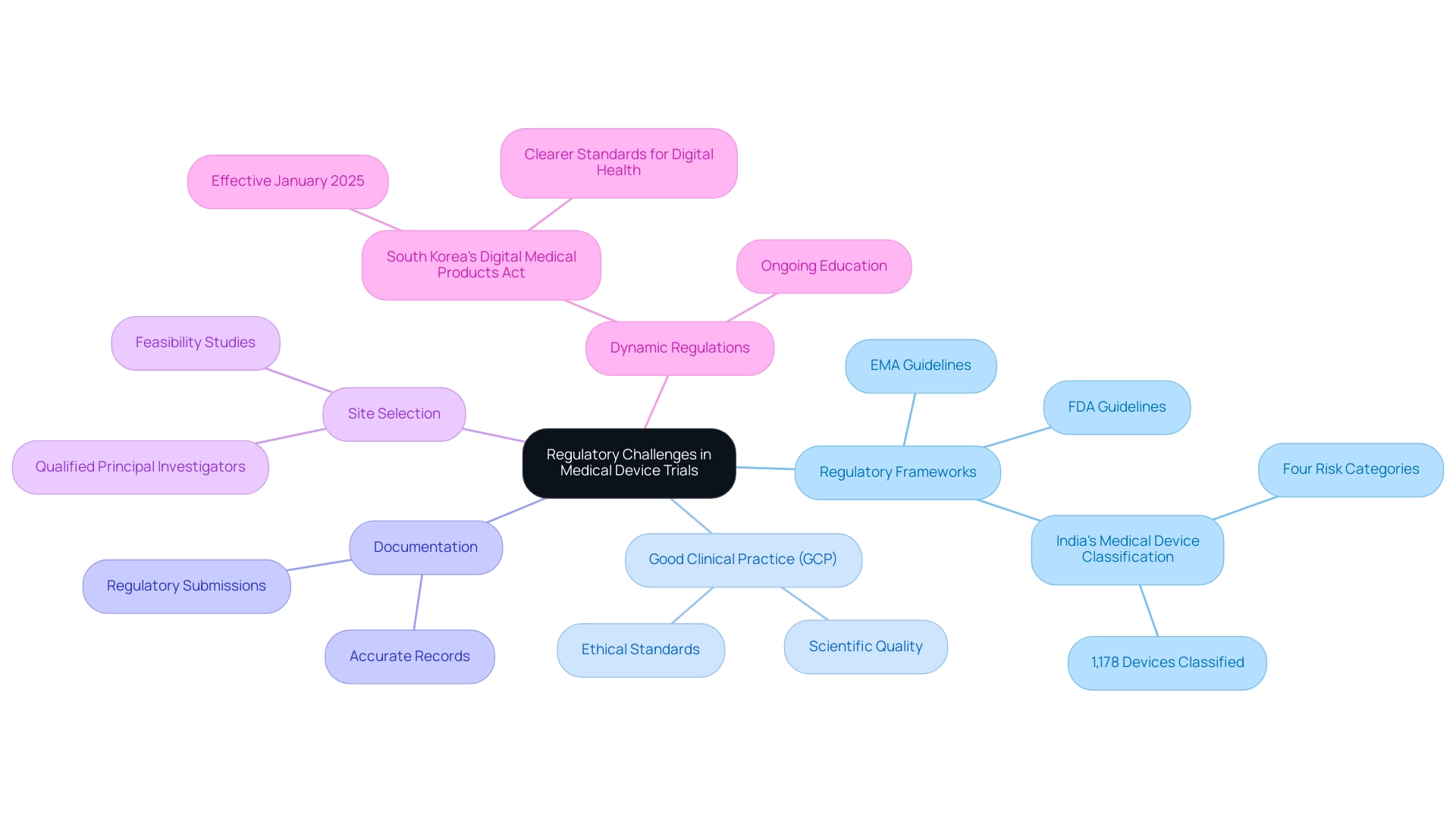
The regulatory landscape for medical device trials is intricate and varies significantly across different regions, presenting several key challenges that organizations must navigate effectively.
Understanding regulatory frameworks is essential. A thorough comprehension of the guidelines established by regulatory bodies such as the FDA and EMA is crucial. These frameworks specify the criteria for research studies and differ according to the classification of the medical device, which can influence the study design and approval process. For instance, India plans to classify approximately 1,178 medical devices into four categories based on their risk profile under the Medical Device Rules, 2017, highlighting the global regulatory landscape.
Compliance with Good Clinical Practice (GCP) is vital for ensuring that clinical studies meet ethical and scientific quality standards. This compliance not only protects participant welfare but also enhances the credibility of the study data, which is essential for regulatory submissions. bioaccess offers comprehensive services that include review and feedback on study documents to ensure compliance with country requirements, thereby facilitating adherence to GCP.
The importance of meticulous documentation cannot be overstated. Accurate and comprehensive records are necessary for regulatory submissions and audits, serving as a foundation for demonstrating compliance with established protocols and guidelines. bioaccess supports this by providing project management and monitoring services that ensure all documentation is properly maintained throughout the testing process.
bioaccess also specializes in the feasibility and selection of research locations and principal investigators (PIs), which is crucial for the success of clinical studies. By identifying suitable sites and qualified PIs, bioaccess helps streamline the study process and enhances the likelihood of successful outcomes.
The regulatory environment is dynamic, with requirements frequently evolving. Organizations must commit to ongoing education and adaptability to stay abreast of these changes. For instance, the recent implementation of South Korea's Digital Medical Products Act in January 2025 has established clearer standards for digital health technologies, facilitating their development and market entry. This act exemplifies how regulatory changes can significantly impact the landscape for medical devices.
Proactively addressing these challenges can significantly streamline testing processes, which is essential for optimizing medical device clinical trials and improving the likelihood of obtaining regulatory approval. As Dr. Oliver Eikenberg, an expert in global medical device regulations, emphasizes, "With limited time to act, now is the moment to take decisive steps toward securing compliance and capitalizing on growth opportunities." This proactive approach is essential, especially as the U.S. Early Feasibility Study (EFS) program continues to support approximately 60 submissions annually, engaging over 4,000 participants and adapting to the evolving needs of the industry in 2025.
Furthermore, regulatory preparedness is essential due to the increasing complexity and demands of regulations, including data privacy considerations. By utilizing best practices and extensive research management services, like those provided by bioaccess, organizations can enhance their strategic positioning in the competitive medtech landscape while optimizing medical device clinical trials to ensure that their studies are not only compliant but also successful.
Best Practices for Designing and Managing Clinical Trials
To optimize clinical trials in the medical device sector, particularly within the Latin American landscape, it is essential to adopt the following best practices:
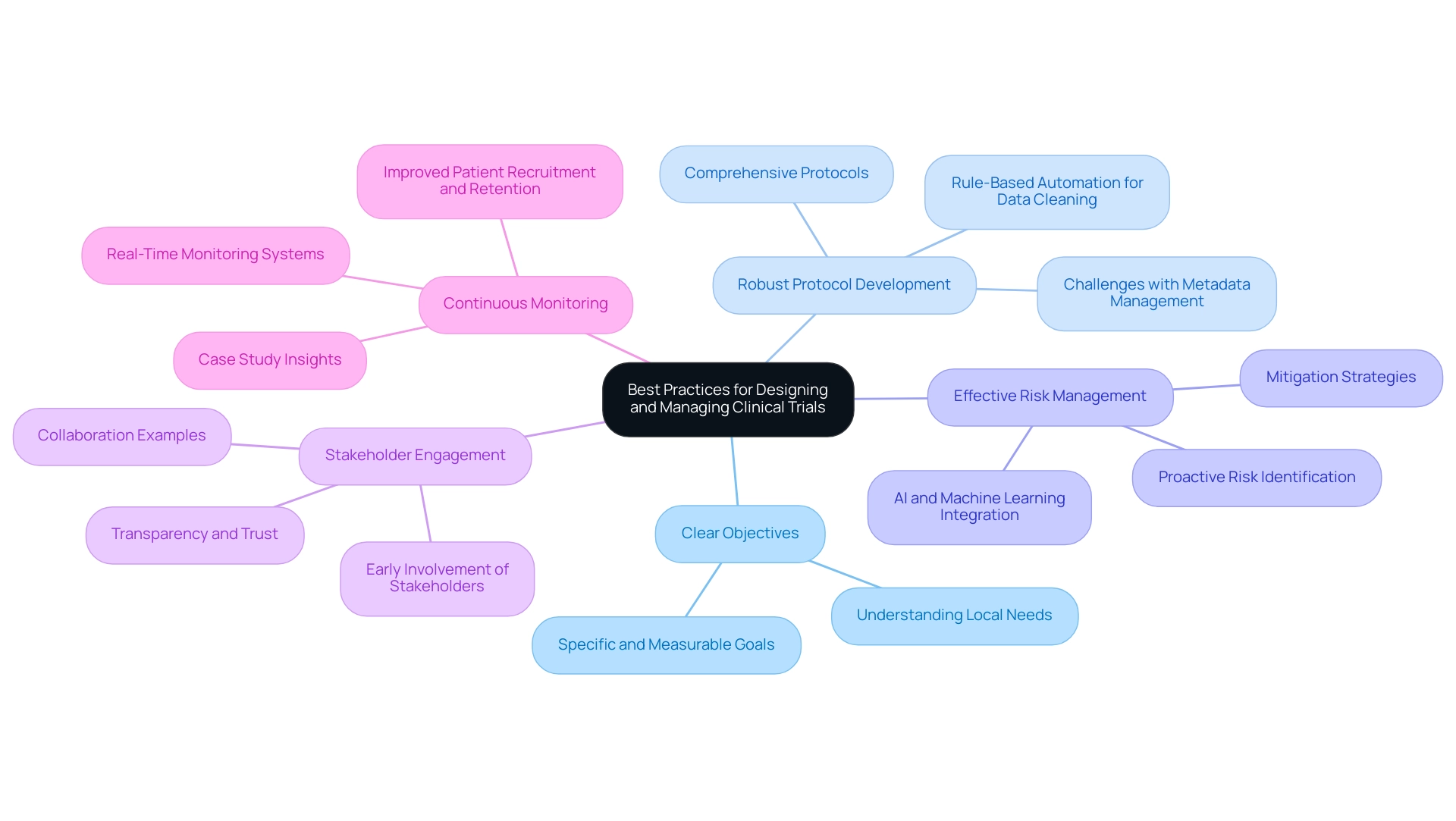
- Clear Objectives: Establish specific and measurable goals that serve as a roadmap for the experiment. Research indicates that studies with well-defined goals are significantly more likely to succeed, with a marked increase in overall effectiveness. This clarity is essential in navigating the complexities of the Latin American market, where understanding local needs can improve results.
- Robust Protocol Development: Create comprehensive protocols that detail methodologies, participant criteria, and information collection methods. This thoroughness not only simplifies the testing process but also improves adherence and information integrity. Companies are increasingly facing challenges with spreadsheets for metadata management, highlighting the need for protocols that address these issues. Additionally, biopharma companies like GSK are utilizing rule-based automation for data cleaning, which accelerates the time to database lock, further emphasizing the importance of robust protocol development.
- Effective Risk Management: Proactively identify potential risks and devise mitigation strategies. Implementing a structured risk management plan can reduce study timelines by up to 30% and costs by as much as 20%, as evidenced by recent advancements in clinical research methodologies. The incorporation of artificial intelligence and machine learning can streamline operations, leading to more efficient assessments, especially in diverse regulatory environments like those in Latin America.
- Stakeholder Engagement: Engage all relevant stakeholders—including patients, investigators, and regulatory bodies—early in the planning process. As Vivienne van der Walle, Founder and Medical Director, states, "Anything that takes away time from patients is a pain point for a site, and anyone who resolves that is helping patient care." This collaborative method encourages transparency and trust, which are essential for successful execution. The partnership between bioaccess and Caribbean Health Group illustrates this strategy, as it aims to improve medical research in Colombia, backed by the Minister of Health.
- Continuous Monitoring: Utilize real-time monitoring systems to ensure adherence to protocols and to address any issues as they arise. This practice not only enhances study integrity but also improves patient recruitment and retention, ultimately leading to more efficient and effective research studies. The case analysis titled "Elevating Site Preparedness: Trends and Strategies for 2025" illustrates how AI and machine learning can reduce timelines and costs, reinforcing the importance of continuous monitoring.
By adopting these best practices, organizations can significantly improve the quality and success rates of their studies, which is crucial for optimizing medical device clinical trials and paving the way for innovative medical devices to reach the market more swiftly and effectively. bioaccess's expertise in managing various study types, including Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, positions it as a leader in facilitating these processes in Latin America.
Leveraging Data Management and Digital Tools in Clinical Trials
Incorporating digital tools and effective data management strategies can significantly transform clinical trials, leading to enhanced efficiency and improved outcomes, particularly in the context of bioaccess®'s comprehensive clinical trial management services in Latin America.
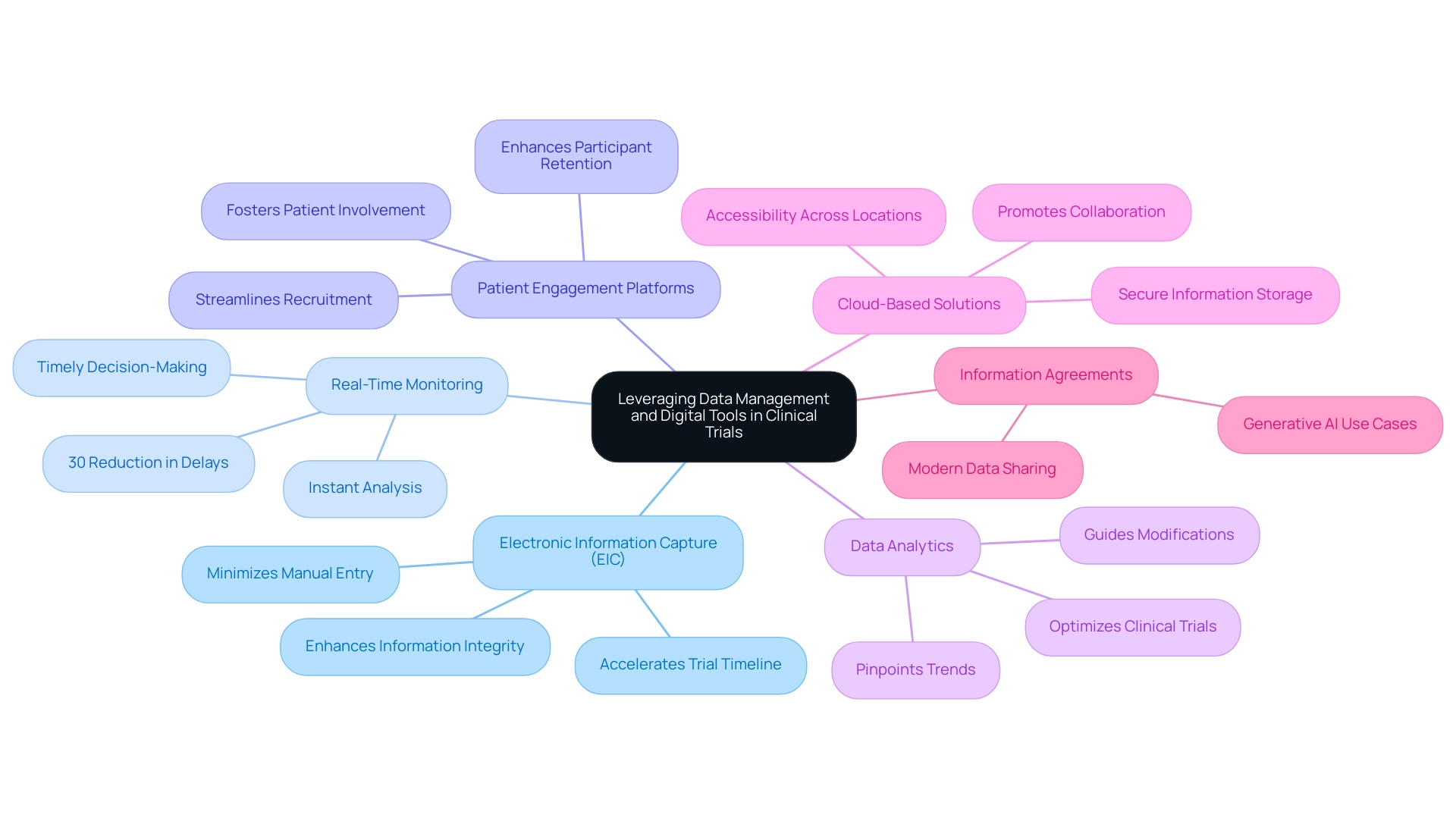
Electronic Information Capture (EIC): The adoption of EIC systems is crucial for streamlining information collection processes. By minimizing manual entry and reducing errors, EDC enhances information integrity and accelerates the overall trial timeline. With spending on EDC technology projected to reach eight trillion U.S. dollars by 2028, the trend underscores its growing importance in clinical research. As Vivienne van der Walle, Founder and Medical Director, states, "Anything that takes away time from patients is a pain point for a site, and anyone who resolves that is helping patient care."
Real-Time Monitoring: Implementing real-time monitoring tools enables instant analysis, facilitating timely decision-making. This capability is crucial for modifying protocols based on new information, ultimately enhancing patient safety and study effectiveness. Statistics indicate that real-time monitoring can lead to a 30% reduction in delays during experiments, showcasing its impact on operational efficiency.
Patient Engagement Platforms: Utilizing digital platforms to enhance communication and engagement with participants is vital. These platforms not only streamline participant recruitment and retention but also foster a sense of involvement among patients, which can lead to higher compliance rates. Involved patients are more likely to provide precise information, further enhancing the quality of the research.
Data Analytics: Utilizing advanced analytics tools allows researchers to pinpoint trends and insights that can guide essential modifications during the study. By examining information in real-time, teams can make informed decisions that are essential for optimizing medical device clinical trials, ultimately leading to more robust results.
Cloud-Based Solutions: The adoption of cloud technologies for secure information storage and accessibility is becoming increasingly important. Cloud solutions promote collaboration among research teams and ensure that information is readily available for analysis, regardless of location. This flexibility is especially advantageous in multi-site studies, where information consistency and security are crucial.
Information Agreements: As a contemporary method for sharing information, information agreements are becoming a crucial element in research trials. They emphasize the strategic deployment of generative AI use cases, enhancing the efficiency of data management strategies.
These strategies not only improve operational efficiency but also enhance the overall quality of medical research, positioning organizations like bioaccess® at the forefront of innovation in the Medtech sector. By connecting innovative medtech firms with the opportunity for conducting research studies in Latin America, bioaccess® plays a vital role in the changing environment of research. Furthermore, the future advancements in EDC technology, including trends towards wearable technology and blockchain integration for information security, suggest a transformative shift in how information is collected and utilized.
Effective Strategies for Patient Recruitment and Retention in Trials
To enhance patient recruitment and retention in research trials, implementing best practices is essential.
- Targeted Outreach: Leverage data analytics to identify and effectively reach potential participants. This approach has demonstrated an increase in recruitment efficiency, with targeted strategies yielding up to a 30% improvement in enrollment rates. With over 20 years of experience in the Medtech industry, bioaccess® understands the intricacies of effective outreach, particularly in expedited medical device research services in Latin America.
- Clear Communication: Providing transparent information regarding the study's objectives, procedures, and potential benefits is crucial. Effective communication nurtures trust and promotes involvement, as emphasized by industry experts like Tiffany Ashton, who highlights that the overall objective for a clinical study is to complete research on schedule, within budget, and with high-quality outcomes. Clarity can significantly enhance participant engagement.
- Incentives: Offering incentives, such as travel reimbursements or complimentary health screenings, can motivate individuals to participate. Research indicates that studies incorporating incentives see a notable increase in participant willingness, which is vital for meeting enrollment targets.
- Flexible Participation Options: Allowing for remote participation or flexible scheduling accommodates diverse participant needs, thereby enhancing recruitment efforts. This flexibility is particularly important in today's fast-paced environment, where convenience can be a deciding factor for potential participants.
- Ongoing Engagement: Maintaining regular communication with participants throughout the study is vital for keeping them informed and engaged. Continuous updates and check-ins help reduce dropout rates, ensuring that participants feel valued and connected to the research. Additionally, investing in leadership development enables managers to effectively support nursing staff and advocate for their needs, which can enhance participant engagement.
- Regulatory Awareness: Given the growing complexity of regulations, staying updated with FDA guidance and international regulations is crucial. This awareness greatly influences patient recruitment and retention approaches, ensuring adherence and cultivating trust among participants.
By applying these methods, studies aimed at optimizing medical device clinical trials can significantly boost enrollment rates and enhance participant retention, ultimately resulting in more successful outcomes. Moreover, partnerships such as bioaccess® with Caribbean Health Group to establish Barranquilla as a premier location for medical research in Latin America, supported by Colombia's Minister of Health, illustrate the positive impact of Medtech health investigations on local economies, including job creation and healthcare enhancement. Promoting supportive policies, as demonstrated in case studies on policy modifications, can create a more advantageous atmosphere for research studies, improving recruitment and retention initiatives.
Furthermore, bioaccess® focuses on Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies, utilizing their vast experience to effectively manage the intricacies of research.
Integrating Real-World Evidence into Clinical Trial Designs
Incorporating real-world evidence (RWE) into clinical study designs presents a vital pathway to achieving more relevant and impactful findings. Key strategies for optimizing this integration include:
-
Utilizing Existing Information: Harnessing data from electronic health records, patient registries, and other pertinent sources can significantly inform trial design. This approach not only enhances the quality of information but also alleviates the burden on participants by allowing researchers to draw insights from pre-existing material. Notably, improving system functionality can help avoid an estimated 54,000 queries a year, further supporting the argument for utilizing existing data.
-
Patient-Centric Approaches: Designing studies that accurately reflect real-world patient populations and their treatment pathways is crucial. By concentrating on the demographics and situations that patients genuinely encounter, studies can yield outcomes that are more relevant to everyday clinical practice. As Bree Burks, Vice President of Site Strategy at Veeva, emphasizes, "Sponsors will step up to solve site capacity issues," highlighting the importance of stakeholder collaboration in this evolving landscape.
-
Adaptive Designs: Implementing adaptive trial designs allows researchers to make adjustments based on real-world information as the trial progresses. This flexibility can lead to more efficient research, enabling modifications that enhance the relevance and applicability of findings. Risk-based approaches can result in higher data quality, greater resource efficiency, and shorter study timelines, making adaptive designs even more valuable.
-
Stakeholder Collaboration: Engaging with various stakeholders—including patients, healthcare providers, and regulatory bodies—ensures that RWE is effectively integrated into study protocols. This collaboration fosters a shared understanding of the study's objectives and enhances the overall quality of the research.
-
Regulatory Alignment: Ensuring that the use of RWE aligns with regulatory expectations and guidelines is essential. As the landscape of medical research evolves, staying informed about regulatory changes can streamline the approval process and enhance the credibility of study outcomes. The trend of sponsors increasingly insourcing data management processes to gain direct control over their data underscores the significance of data ownership and transparency in the evolving research environment.
By integrating RWE into medical studies, researchers can improve patient outcomes through the optimization of medical device clinical trials, generating results that align more closely with real-world settings. Moreover, as Max Baumann from Tree Hill Partners cautions, the medical markets are becoming crowded, presenting fundamental business model challenges for biotech. This reality emphasizes the necessity for innovative study designs that incorporate RWE while optimizing medical device clinical trials to remain competitive.
Future Trends in Medical Device Clinical Trials: Innovations and Insights
The landscape of medical device clinical trials is undergoing significant transformation, driven by several pivotal trends that are reshaping the industry:
-
Artificial Intelligence and Machine Learning: These advanced technologies are transforming testing procedures by automating information assessment and enhancing patient recruitment strategies. In 2025, it is projected that AI will enhance the efficiency of clinical studies, with statistics indicating a potential reduction in timelines by up to 30%. Notably, 45% of Alcon's data is entered on the same day as the visit date, showcasing the efficiency that can be achieved through effective data management.
-
Decentralized Studies: The shift towards decentralized and hybrid research models is gaining momentum, significantly increasing accessibility and participant diversity. This approach not only facilitates broader patient engagement but also aligns with the FDA's recent guidance encouraging designs that reflect routine clinical practice, thereby enhancing enrollment and participation rates.
-
Patient-Centric Designs: A growing emphasis on patient needs and preferences is leading to the development of more engaging trial protocols. As Vivienne van der Walle, Founder and Medical Director, states, "Anything that takes away time from patients is a pain point for a site, and anyone who resolves that is helping patient care." By incorporating feedback from participants, organizations can enhance retention rates and overall satisfaction, ultimately contributing to more robust information collection.
-
Regulatory Innovations: Regulatory bodies are evolving alongside technological advancements, adapting their frameworks to accommodate new methodologies. The resurgence of Medical Device Regulation (MDR) emphasizes the importance of information standards in study design, collection, analysis, and submission. This shift is creating opportunities for expedited approvals, allowing innovative medical devices to reach the market more swiftly.
-
Integration of Digital Health Technologies: The incorporation of wearables and mobile health applications is providing real-time information, enhancing participant monitoring and engagement. This integration not only enhances data precision but also enables patients to take an active role in their health management during studies.
Furthermore, bioaccess® provides extensive research management services, including feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting. By utilizing their knowledge in Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, bioaccess® ensures that studies are conducted efficiently and effectively.
Some sponsors are also utilizing historical trend information to address issues proactively during research studies. By defining thresholds and sharing data across departments, they can monitor trends and document remediation efforts, enhancing data quality and leading to faster approvals, greater resource efficiency, and shorter study timelines.
Staying informed about these trends is crucial for organizations aiming at optimizing medical device clinical trials to enhance patient outcomes. By leveraging these advancements, clinical research can become more efficient, patient-focused, and ultimately more successful.
Conclusion
Medical device clinical trials are essential for ensuring the safety and efficacy of innovative technologies. Understanding the various trial types—from pilot studies that establish feasibility to pivotal studies that secure regulatory approval—is crucial for stakeholders, particularly in emerging markets like Latin America, where robust trial design and operational efficiency are paramount.
Organizations must navigate a complex regulatory landscape that varies by region. Adhering to Good Clinical Practice and maintaining meticulous documentation can streamline processes and enhance the likelihood of regulatory approval. Proactively addressing these challenges strengthens a company’s position in the competitive medtech sector.
Implementing best practices such as:
- Setting clear objectives
- Developing comprehensive protocols
- Utilizing real-time monitoring
can significantly improve trial outcomes. The integration of digital tools and effective data management strategies enhances operational efficiency, leading to better patient engagement and data integrity. Furthermore, incorporating real-world evidence into trial designs produces more relevant findings that can positively influence patient care.
Looking ahead, trends such as:
- Artificial intelligence
- Decentralized trials
- Patient-centric approaches
are reshaping the clinical trial landscape. By adapting to these innovations, organizations can enhance patient outcomes and ensure that groundbreaking medical devices reach the market efficiently and safely. The future of medical device clinical trials is promising, and stakeholders are well-positioned to drive meaningful advancements in healthcare.
Frequently Asked Questions
What are the different types of clinical studies for medical devices?
The types of clinical studies for medical devices include Pilot Studies, First-in-Human Trials (FIH), Early-Feasibility Assessments (EFA), Pivotal Research, and Post-Market Clinical Follow-Up Assessments (PMCF). Each type serves a unique purpose in evaluating the safety and efficacy of medical devices.
What is the purpose of Pilot Studies in medical device trials?
Pilot Studies are early-stage trials that assess feasibility and gather preliminary data. They help identify potential challenges and refine research protocols, which can significantly reduce the risk of failure in later stages.
What do First-in-Human Trials (FIH) involve?
First-in-Human Trials (FIH) are the first experiments involving human subjects, focusing on evaluating the safety profile of new medical devices and providing preliminary information on how the device interacts with the human body.
What is the significance of Early-Feasibility Assessments (EFA)?
Early-Feasibility Assessments (EFA) gather initial safety and performance data from a small group of patients. They are crucial for determining whether a device is ready for larger-scale pivotal evaluations.
What does Pivotal Research aim to achieve?
Pivotal Research aims to provide conclusive proof of a device's efficacy and safety through large-scale trials, which are essential for regulatory approval and demonstrating compliance with regulatory standards.
What are Post-Market Clinical Follow-Up Assessments (PMCF)?
Post-Market Clinical Follow-Up Assessments (PMCF) are conducted after a device is introduced to the market to monitor long-term safety and effectiveness, ensuring ongoing compliance with regulatory requirements.
Why is understanding regulatory frameworks important for medical device trials?
Understanding regulatory frameworks is crucial as they dictate the criteria for research studies, which can vary based on the classification of the medical device and influence study design and approval processes.
What role does Good Clinical Practice (GCP) play in clinical studies?
Compliance with Good Clinical Practice (GCP) ensures that clinical studies meet ethical and scientific quality standards, protecting participant welfare and enhancing the credibility of study data for regulatory submissions.
How does bioaccess support compliance and documentation in clinical trials?
Bioaccess provides comprehensive services for reviewing study documents to ensure compliance with country requirements and offers project management and monitoring services to maintain accurate documentation throughout the testing process.
What is the importance of identifying suitable research locations and principal investigators (PIs)?
Identifying suitable research locations and qualified principal investigators (PIs) is critical for the success of clinical studies, as it streamlines the study process and enhances the likelihood of successful outcomes.
How are regulatory requirements evolving in the medical device industry?
Regulatory requirements are dynamic and frequently changing, necessitating ongoing education and adaptability for organizations to stay compliant and capitalize on growth opportunities, such as the implementation of new acts like South Korea's Digital Medical Products Act.
What is the significance of regulatory preparedness in medical device trials?
Regulatory preparedness is essential due to the increasing complexity of regulations, including data privacy considerations, and it helps organizations enhance their strategic positioning and optimize clinical trials for compliance and success.

