Overview
The article presents an authoritative overview of the regulatory considerations vital for designing clinical trials, clearly stating its purpose and relevance to clinical research. It emphasizes the necessity of compliance with guidelines established by agencies such as the FDA and EMA. By detailing how adherence to Good Clinical Practice (GCP) and ethical standards enhances participant safety and data quality, the article builds interest and generates desire for rigorous trial designs. Furthermore, it reinforces conviction by illustrating that such compliance significantly improves the likelihood of successful study outcomes and regulatory approvals.
In conclusion, the article underscores the importance of collaboration in navigating these regulatory landscapes, prompting action towards improved clinical trial practices.
Introduction
In the intricate world of clinical trials, regulatory frameworks serve as the backbone of ethical and effective research practices. With pivotal authorities like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) at the helm, these regulations ensure that trials are designed and conducted with the utmost safety and integrity.
As the landscape evolves, particularly with the introduction of decentralized clinical trials and the emphasis on patient engagement, understanding these frameworks becomes increasingly vital for researchers.
This article delves into the complexities of regulatory compliance, the impact of digital technologies, and the ethical considerations that shape modern clinical trial design, all while highlighting best practices that can lead to successful outcomes in this competitive arena.
Understanding Regulatory Frameworks in Clinical Trials
Regulatory considerations for trial design represent fundamental elements established by various agencies, particularly the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA). These considerations govern the framework for the design, execution, and reporting of research studies, ensuring compliance with stringent safety and ethical standards. Central to these regulations is Good Clinical Practice (GCP), which safeguards the rights and welfare of participants, alongside the Declaration of Helsinki, which delineates ethical principles for medical research involving human subjects.
As we approach 2025, notable updates to research regulations have emerged, particularly with the FDA's Final Guidance on decentralized studies released in September 2024. This guidance underscores a growing trend towards more adaptable study designs that enhance participant access and diversity, addressing the imperative for underrepresented populations to have greater choices in onboarding and participation.
Statistics reveal that adherence to GCP not only bolsters participant safety but also elevates the quality of data collected, which is vital for compliance submissions. Research indicates that experiments designed with GCP principles in mind are 30% more likely to achieve successful results and approvals compared to those that do not adhere to these standards.
A significant case study highlighting this is Lilly's commitment to patient access, which has been enhancing diversity in research through community-based studies since 2002. Their accelerated initiatives during the Covid-19 pandemic underscore the effectiveness of this approach in improving patient access and outcomes. Lilly's experience illustrates the necessity for ongoing collaboration and policy updates to standardize community-based studies in medical research, aligning with compliance and risk management strategies.
In light of the increasingly saturated medical markets, as noted by Max Baumann from Treehill Partners, 'Entering 2025, we observe biotech encountering essential business model challenges as medical end-markets become increasingly crowded.' This observation emphasizes the importance of refining development journeys while adhering to legal frameworks for commercial success. Understanding these frameworks not only aids researchers in aligning their experimental designs with regulatory considerations for trial design, but also facilitates smoother approvals and compliance throughout the research lifecycle.
At bioaccess, we provide comprehensive management services for medical experiments, encompassing:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project management
- Reporting
Our offerings also include thorough reviews and feedback on study documents to ensure compliance with country requirements and reporting on serious and non-serious adverse events. Our team, featuring professionals such as Ana Criado, Director of Compliance Affairs, and Katherine Ruiz, an expert in Compliance Affairs for Medical Devices and In Vitro Diagnostics, is dedicated to ensuring that your clinical studies meet all compliance standards while fostering innovation and excellence in the Medtech industry across Latin America.
Key Considerations for Medical Device Trial Design
Designing trials for medical devices necessitates a comprehensive understanding of regulatory considerations for trial design. Central to this process is the classification of the device, which plays a crucial role in determining the appropriate regulatory pathway. For significant risk devices, obtaining an Investigational Device Exemption (IDE) is essential, as it permits the investigation of devices that may pose a higher risk to patients.
As we look to 2025, successful IDE applications increasingly rely on robust data management practices, underscoring the importance of operationalizing research in-house to maintain control over data quality and integrity. The Head of Clinical Data Engineering notes, "Traditionally, data management was outsourced to our CRO vendor partners. Part of the initiative is to bring all our research in-house so that our internal teams can start working on it. They can be more hands-on, and we operationalize studies in-house and we are able to take control of our data, and we deliver for our patients with high quality."
Moreover, study designs must meticulously define endpoints pertinent to both device efficacy and safety. This encompasses not only primary outcomes but also secondary endpoints that may inform post-market surveillance strategies. Engaging with regulatory bodies early in the design process is vital, as it facilitates a clearer understanding of requirements and can significantly streamline the approval process.
Statistics reveal that the success rates of medical studies remain challenging, with only about 5-14% of treatments completing all phases and obtaining approval. This highlights the rigorous evaluation process necessary to ensure patient safety and treatment efficacy. Additionally, the new FDA draft guidance on Diversity Action Plans encourages sponsors to enroll more patients from underrepresented groups, emphasizing the significance of diversity in research studies.
As the landscape of medical device evaluations evolves, staying informed about regulatory considerations for trial design—including the latest IDE requirements and compliance factors—is crucial for achieving successful research outcomes.
At bioaccess®, with over 20 years of experience in Medtech, we focus on comprehensive management services for research in Latin America. Our offerings include:
- Feasibility assessments
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting
Our expertise spans Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, ensuring that your medical investigations are designed and executed with the utmost precision and care. The case analysis titled 'Success Rates and Statistics' illustrates the rigorous nature of medical research, emphasizing the necessity for thorough evaluation processes.
Ethical Considerations in Clinical Trial Design
Ethical considerations in research design are paramount, encompassing the necessity of obtaining informed consent, safeguarding participant confidentiality, and balancing risks with potential benefits. Adhering to established ethical guidelines, such as those outlined in the Belmont Report, is essential. This report emphasizes three core principles: respect for persons, beneficence, and justice, which serve as a foundation for ethical research practices.
At bioaccess, our comprehensive clinical study management services support these ethical considerations through meticulous feasibility studies and site selection processes. We ensure that study protocols undergo thorough compliance reviews, facilitating setup, start-up, and obtaining necessary import permits in accordance with country requirements. This structured approach not only adheres to ethical standards but also fosters an environment of trust and transparency, crucial for participant engagement and stakeholder confidence.
Institutional Review Boards (IRBs) are essential to this process, as they meticulously examine research protocols to ensure compliance with ethical standards. Their oversight helps to foster an environment of trust and transparency, which is crucial for participant engagement and stakeholder confidence.
Informed consent processes must be clear and comprehensive, allowing participants to make educated decisions about their involvement. Recent statistics indicate that approximately 70% of participants in clinical trials express a desire for more information regarding the risks and benefits associated with their participation. This highlights the need for researchers to prioritize effective communication during the consent process.
Moreover, ethical considerations extend beyond consent. A notable case analysis examined the ethical implications of expanding diabetic retinopathy screening through artificial intelligence (AI) without ensuring access to necessary treatments. Participants in this study underscored the importance of addressing treatment access alongside screening initiatives to prevent ethical dilemmas and ensure that AI interventions genuinely benefit underserved populations.
One participant remarked, "If the AI is efficacious, it’s an accurate diagnostic, but is this cost-effective if it costs a million dollars to run?"
Incorporating these ethical considerations from the outset not only enhances the integrity of research studies but also promotes compliance and trust among participants and stakeholders alike. As the landscape of medical research evolves, maintaining a strong commitment to ethical standards remains crucial for the advancement of medical technology. Furthermore, the fragility of moral progress in research ethics necessitates strong commitments from international codes and professional societies, as highlighted in discussions from The Lancet Global Health Series on Clinical Trials.
By utilizing our knowledge in project management and reporting, bioaccess guarantees that ethical standards are maintained throughout the process, ultimately aiding in job creation, economic growth, and healthcare enhancement in local economies.
The Role of Patient Engagement in Trial Design
Patient involvement has emerged as a cornerstone of efficient research design, significantly influencing both recruitment and retention rates. By actively engaging patients in discussions about study objectives, design, and outcomes, researchers can obtain invaluable insights that enhance the study's relevance and acceptability. In 2025, the incorporation of patient viewpoints is expected to further elevate the quality of medical studies, as advancements in biomarker identification and validation will enhance disease diagnosis and treatment assessment.
The collaboration between bioaccess™ and Caribbean Health Group aims to position Barranquilla as a leading hub for medical studies in Latin America, supported by Colombia's Minister of Health. This initiative not only transforms the clinical research landscape but also underscores the critical role of patient involvement in achieving successful study outcomes. Effective patient recruitment strategies encompass diverse approaches, including:
- Focus groups
- Surveys
- The integration of patient advocates in the planning process
These methodologies not only foster a sense of ownership among participants but also ensure that the study design aligns with patient needs and expectations. For example, case studies have shown that studies integrating patient feedback during the design phase experience enhanced recruitment rates and reduced dropout rates, ultimately leading to more robust data collection.
Statistics reveal that studies employing strong patient engagement strategies can witness recruitment rates soar by up to 30%, highlighting the necessity of prioritizing patient perspectives. Additionally, as sponsors increasingly seek to insource data management processes for improved control and transparency, the significance of patient engagement in shaping study outcomes becomes even more pronounced. This trend emphasizes the imperative for researchers to maintain direct access to live data, ensuring that patient involvement is not only acknowledged but also effectively woven into the study process.
Moreover, the partnership with GlobalCare Clinical Trials has demonstrated the potential for remarkable improvements in research operations, achieving over a 50% reduction in recruitment time and 95% retention rates. This case analysis illustrates the need for early-stage drug developers to factor in commercial outcomes within their strategies. By focusing on patient participation, researchers can refine their study designs, which must also accommodate regulatory considerations for trial design, paving the way for successful regulatory approvals and commercial viability in an increasingly competitive landscape.
As Peng Lu, chief medical officer of Dutch biotech Pharvaris, articulated, "Harmonizing the use of specific outcomes and outcome measures for trials will aid in the development of guidelines and future indirect comparisons among interventions." This statement emphasizes the significance of patient viewpoints in study design, further reinforcing the case for heightened patient involvement.
Ensuring Compliance with Regulatory Standards
Ensuring adherence to regulatory standards is essential in research design and involves regulatory considerations for trial design, necessitating a deep understanding of applicable laws and guidelines, including Good Clinical Practice (GCP) and local regulations. At bioaccess, our extensive clinical research management services encompass:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project oversight
- Reporting
This includes the review and feedback on research documents to meet country requirements, all of which are critical for maintaining compliance throughout the procedure.
Key strategies for maintaining compliance include the creation of a robust research protocol, which serves as the foundation for all project activities. Regular training for research staff is crucial; studies indicate that effective training can significantly enhance compliance rates. Statistics show that organizations with comprehensive training programs experience up to a 25% reduction in compliance-related issues.
Implementing quality assurance measures is another essential strategy. This involves performing regular audits and monitoring to identify areas for enhancement and ensure compliance with regulatory requirements during the study. The integration of diverse data sources is increasingly vital for modern research methods, necessitating streamlined data flows that can enhance compliance and operational efficiency.
By 2025, AI tools are anticipated to manage up to 50% of data-related activities in medical studies, further highlighting the significance of technology in ensuring compliance.
Moreover, maintaining open communication with regulatory authorities can facilitate timely feedback, allowing for the proactive resolution of potential compliance issues before they escalate. Innovations in medical studies are also simplifying the site experience, with user-friendly systems designed to enhance data entry efficiency and lessen the load on research personnel.
A recent case analysis highlighted by the Tufts Center for the Study of Drug Development illustrates the impact of integrating artificial intelligence and machine learning in clinical trials. These technologies have been shown to reduce study timelines by up to 30% and costs by as much as 20%, while also improving patient recruitment and retention. As noted by the Head of Clinical Data Engineering, "Traditionally, data management was outsourced to our CRO vendor partners. Part of the initiative is to bring all our research in-house so that our internal teams can start working on it. They can be more hands-on, and we operationalize studies in-house, allowing us to take control of our data and deliver high quality for our patients." Such advancements not only streamline operations but also help maintain adherence to standards.
As we approach 2025, the focus on GCP compliance strategies will continue to evolve, emphasizing the use of technology and training to improve the overall quality of research studies. By adopting these strategies, organizations can ensure that their testing protocols align with regulatory considerations for trial design while also contributing to the successful advancement of medical technologies.
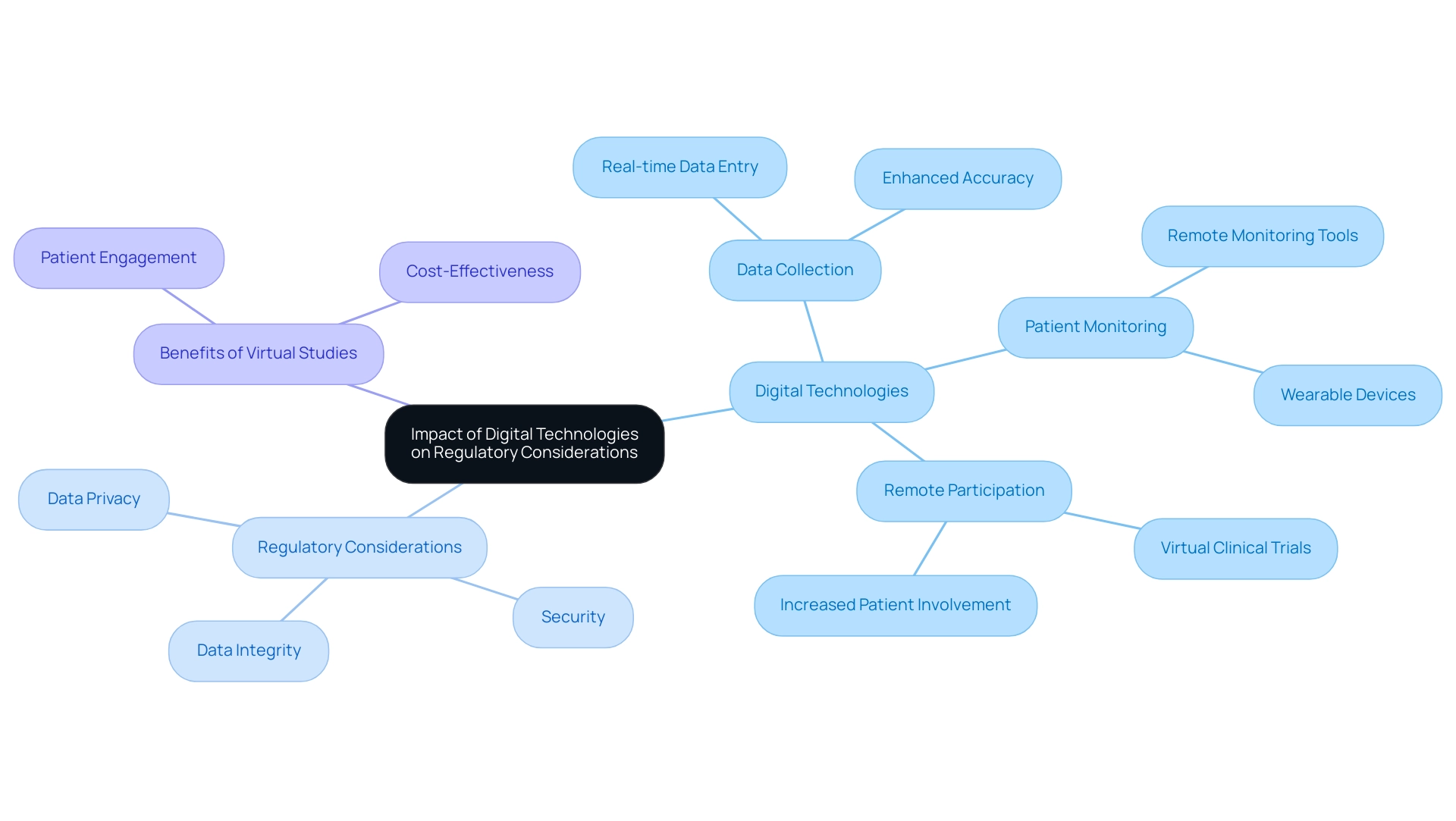
Impact of Digital Technologies on Regulatory Considerations
Digital technologies are revolutionizing research design and execution, paving new avenues for data collection, patient monitoring, and remote participation. By 2025, the integration of these technologies has significantly enhanced trial efficiency and patient engagement. Recent statistics reveal that 45% of data from major research studies is now entered on the same day as the visit date, illustrating the speed and accuracy that digital tools can deliver.
However, the swift adoption of digital technologies also introduces regulatory considerations for trial design, particularly regarding data privacy, security, and integrity. Regulatory organizations are actively updating their guidelines to address these challenges, underscoring the necessity for robust data management systems and clearly defined procedures for employing digital tools in research studies. This evolution is evident as sponsors increasingly assume control of their data management processes, transitioning from outsourcing to insourcing to improve quality and oversight.
As the Head of Clinical Data Engineering remarked, "Traditionally, data management was outsourced to our CRO vendor partners. Part of the initiative is to bring all our research in-house so that our internal teams can start working on it. They can be more hands-on, and we operationalize studies in-house, allowing us to take control of our data and deliver high quality for our patients."
Moreover, the rise of virtual studies exemplifies how technology can enhance patient retention and reduce costs compared to conventional methods. The concept of virtual studies is gaining traction as a means to boost patient involvement and facilitate remote participation and data collection. These studies not only demonstrate significant advantages in patient engagement and cost-effectiveness but also serve as a practical illustration of how digital technologies can transform medical research.
As researchers navigate this evolving landscape, staying informed about the regulatory considerations for trial design related to digital technologies is paramount. This knowledge will empower them to ensure compliance while fully leveraging the benefits that these innovations can provide to their research. Furthermore, improved biomarker identification and validation will advance disease diagnosis and therapeutic evaluation, further highlighting the importance of integrating digital technologies into study design.
At bioaccess, our comprehensive research study management services encompass:
- Feasibility studies
- Site selection
- Compliance assessments
- Study setup
- Import permits
- Project management
- Reporting
Compliance assessments are vital in ensuring that all aspects of the experiment align with the latest legal standards, emphasizing the regulatory considerations for trial design as these regulations evolve. With experts like Katherine Ruiz overseeing our operations for medical devices and in vitro diagnostics in Colombia, we are well-equipped to navigate the complexities of research studies while ensuring compliance with regulatory considerations for trial design and adherence to changing rules.
This holistic approach not only elevates the quality of our studies but also contributes to local economies through job creation, economic growth, and improved healthcare outcomes.
Regional Regulatory Challenges in Clinical Trials
Carrying out research studies in Latin America requires navigating a complex oversight framework that encompasses regulatory considerations for trial design, varying requirements across nations, language barriers, and differing ethical norms. In 2025, researchers face significant compliance challenges, particularly due to the necessity of adapting to country-specific regulations, which can differ markedly from one nation to another. For instance, Colombia has introduced incentives such as tax reductions and government funding to attract more research projects, transforming it into an increasingly appealing destination for medical studies in the region.
Notably, ReGelTec's Early Feasibility Study on HYDRAFIL™ for addressing chronic low back pain in Barranquilla exemplifies the potential for successful clinical studies in Colombia, bolstered by local partnerships like that between bioaccess and Caribbean Health Group, which aim to establish the region as a prominent center for clinical research in Latin America.
To effectively navigate these intricacies, it is essential for researchers to engage local compliance specialists who possess a comprehensive understanding of the regulatory considerations for trial design specific to each nation involved in the study. Building robust relationships with local regulatory authorities is crucial, as these considerations can facilitate smoother approval processes and enhance compliance, ultimately reducing the time to market for innovative medical devices. The collaboration between 'GlobalCare Clinical Trials' and bioaccess has demonstrated substantial improvements in ambulatory services for research in Colombia, achieving over a 50% reduction in recruitment duration and 95% retention rates, showcasing the efficacy of thorough management services for research projects, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project management
- Reporting
Furthermore, cultural differences significantly influence patient perceptions of medical recommendations, complicating informed consent processes. This is particularly relevant in a region where literacy rates, such as Chile's at 95.7%, underscore the necessity for clear communication. As Saisuman Revankar aptly states, "Clear communication is critical for navigating the complexities of clinical studies, ensuring that all parties remain aligned."
Customizing study designs to accommodate these regional differences is vital, especially considering the regulatory considerations for trial design. This approach not only enhances participant recruitment and retention but also leads to more successful outcomes.
As the governance landscape continues to evolve, addressing challenges such as insufficient preparation and underrepresentation of patients will be essential in meeting the regulatory expectations for trial design across diverse populations in Latin America. Policies that concentrate on these issues will improve the overall effectiveness of trials in the region, supported by the oversight of INVIMA, Colombia's National Food and Drug Surveillance Institute, which plays a critical role in medical device regulation as a Level 4 health authority by PAHO/WHO.
Best Practices for Navigating Regulatory Landscapes
Navigating the regulatory considerations for trial design in medical research requires a strategic approach, particularly as the sector evolves in 2025. Key best practices include:
- Staying informed, which is crucial for clinical research teams to remain updated on current regulations and guidelines. This foundational knowledge is essential for ensuring compliance and adapting to any changes that may arise.
- Engaging with oversight authorities early in the study design process can significantly streamline the approval process. This proactive engagement fosters a clearer understanding of expectations and requirements, ultimately enhancing the likelihood of success.
- Fostering strong partnerships among stakeholders—including sponsors, oversight bodies, and clinical sites—can strengthen regulatory considerations for trial design, leading to more effective study designs. Collaborative efforts enable the sharing of insights and resources, which is vital for navigating complex compliance environments.
- Developing comprehensive training initiatives for research staff ensures that all team members are well-versed in their roles and responsibilities regarding compliance. This not only enhances adherence to regulations but also promotes a culture of accountability within the research team.
- Continuous evaluation and updating of study protocols in response to regulatory considerations for trial design are vital practices. This diligence helps maintain compliance and adapt to new requirements, thereby safeguarding the integrity of the trial.
In 2025, research sponsors are expected to adhere to a core outcome set across various designs. This emphasis on standardizing outcome measures will aid in developing guidelines and facilitating indirect comparisons among interventions. Such a shift underscores the importance of standardizing patient-reported outcomes and biomarker identification, as highlighted in recent discussions among experts. This standardization is crucial for enhancing the assessment of drug effectiveness and safety, especially in intricate therapeutic fields, as demonstrated in the case study titled 'Measuring Outcomes,' which emphasizes these elements in research.
Additionally, as the biotech landscape becomes increasingly crowded, early-stage developers must concentrate on commercial outcomes in their strategies. Max Baumann, Head of Execution at Tree Hill Partners, noted, "We expect continued focus on optimizing the development journeys of assets to achieve not only an approval-enabling endpoint but to qualify for commercial success." Insights from industry leaders highlight the necessity of enhancing development journeys to reach endpoints that facilitate approval and position products for commercial success.
By following these best practices and promoting collaboration, research teams can improve their compliance and risk management strategies, incorporating regulatory considerations for trial design, ultimately aiding in the success of their studies.
At bioaccess, our service offerings encompass:
- Feasibility studies
- Site selection
- Compliance assessments
- Trial preparation
- Import permits
- Project management
- Thorough reporting on study status and adverse occurrences
We ensure that your trials are executed effectively and in accordance with all legal requirements. Our methodologies focus on integrating the latest regulatory insights and best practices, particularly regarding regulatory considerations for trial design, to support your clinical research objectives.
Conclusion
Regulatory frameworks are paramount to the integrity and success of clinical trials, ensuring adherence to stringent ethical standards and the safeguarding of participant rights. As the landscape evolves—particularly with advancements in decentralized trials and digital technologies—it is essential for researchers to remain informed and compliant with the latest regulations. The integration of patient engagement strategies alongside robust data management practices enhances the quality and relevance of trial outcomes, illustrating that the journey to successful approvals transcends mere regulatory compliance; it is also about cultivating trust and transparency.
As we approach 2025, the focus on ethical considerations, compliance with Good Clinical Practice (GCP), and proactive engagement with regulatory authorities will be critical. Insights drawn from case studies, including community-based trials and the application of artificial intelligence, highlight the necessity of adapting to the unique challenges posed by diverse regulatory environments, particularly in regions such as Latin America.
Ultimately, navigating the complexities of clinical trial regulations demands a collaborative approach among stakeholders, continuous education, and a steadfast commitment to ethical research practices. By embracing these principles and leveraging cutting-edge technological advancements, researchers can not only bolster their compliance and operational efficiency but also contribute to the broader advancement of medical technologies and improved patient outcomes in an ever-evolving landscape.
Frequently Asked Questions
What are the key regulatory agencies involved in trial design?
The key regulatory agencies involved in trial design are the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
What is Good Clinical Practice (GCP)?
Good Clinical Practice (GCP) is a set of guidelines that ensures the rights and welfare of participants in clinical trials, as well as the integrity of the data collected.
What is the Declaration of Helsinki?
The Declaration of Helsinki is a set of ethical principles for medical research involving human subjects, aimed at ensuring ethical standards are upheld in clinical trials.
What notable updates to research regulations were highlighted for 2025?
Notable updates include the FDA's Final Guidance on decentralized studies, which promotes more adaptable study designs to enhance participant access and diversity.
How does adherence to GCP impact research studies?
Adherence to GCP enhances participant safety and improves the quality of data collected, making studies designed with GCP principles 30% more likely to achieve successful results and approvals.
Can you provide an example of a company enhancing diversity in research?
Lilly has been enhancing diversity in research through community-based studies since 2002, with accelerated initiatives during the Covid-19 pandemic to improve patient access and outcomes.
What challenges are anticipated in the biotech industry as we approach 2025?
The biotech industry is expected to face essential business model challenges due to increasingly crowded medical end-markets, necessitating refined development journeys and adherence to legal frameworks.
What services does bioaccess provide for medical experiments?
Bioaccess provides comprehensive management services including feasibility assessments, site selection, compliance evaluations, setup, import permits, project management, and reporting.
What is an Investigational Device Exemption (IDE)?
An Investigational Device Exemption (IDE) is a regulatory approval that allows the investigation of significant risk devices that may pose a higher risk to patients.
Why is data management important for successful IDE applications?
Robust data management practices are crucial for successful IDE applications as they ensure control over data quality and integrity, which is vital for maintaining high standards in research.
What is the significance of engaging with regulatory bodies early in the trial design process?
Engaging with regulatory bodies early helps clarify requirements and can significantly streamline the approval process for clinical trials.
What does the new FDA draft guidance on Diversity Action Plans emphasize?
The new FDA draft guidance emphasizes the importance of enrolling more patients from underrepresented groups in clinical research studies to enhance diversity.
What types of studies does bioaccess specialize in?
Bioaccess specializes in Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies in the Medtech sector.

