Overview
The article focuses on the top clinical research organizations (CROs) that are essential for the planning and execution of medical studies, highlighting their roles in regulatory compliance, project management, and patient recruitment. It emphasizes that these organizations, such as bioaccess®, provide specialized expertise that helps streamline research processes and address challenges faced by medical device companies, thereby significantly enhancing the efficiency and effectiveness of clinical trials.
Introduction
The landscape of clinical research is evolving rapidly, shaped by the increasing complexity of medical innovations and the critical need for effective trial management. Clinical Research Organizations (CROs) have emerged as essential partners in this journey, providing a diverse range of services that streamline the clinical trial process while ensuring compliance with rigorous regulatory standards.
As the demand for specialized expertise grows, particularly in regions like Latin America, CROs are not only facilitating the logistical aspects of trials but also addressing significant challenges faced by medical device companies, such as:
- Regulatory hurdles
- Patient recruitment
This article delves into the multifaceted role of CROs, highlighting their contributions to:
- Enhancing trial efficiency
- Promoting diversity in research
- Leveraging cutting-edge technology to transform the clinical research landscape
Through insightful examples and emerging trends, it becomes evident that CROs are pivotal in advancing medical research and improving health outcomes globally.
Understanding the Role of Clinical Research Organizations (CROs)
The best clinical research organizations contribute significantly to the planning, execution, and management of studies, providing a complete range of services that include:
- Feasibility and selection of study site and principal investigator (PI)
- Regulatory compliance
- Project management
- Reporting
By partnering with pharmaceutical, biotechnology, and medical device firms, CROss enable a streamlined research process, ensuring studies are performed effectively and conforming rigorously to regulatory standards. This capability is particularly critical, as medical device startups often face significant challenges such as:
- Regulatory hurdles
- Competition
- Recruitment issues
- Financial constraints
A significant instance is the partnership between bioaccess™ and Caribbean Health Group, which seeks to establish Barranquilla as a premier location for medical studies in Latin America, backed by Colombia's Minister of Health. Furthermore, GlobalCare Clinical Studies has enhanced clinical study ambulatory services in Colombia through its partnership with bioaccess™, achieving over a 50% reduction in recruitment time and a remarkable 95% retention rate. These advancements highlight the significance of the best clinical research organizations in easing the burden for sponsors, enabling them to focus on core activities while gaining from specialized expertise.
Furthermore, the worldwide scope of the best clinical research organizations allows access to varied patient groups and navigation of intricate regulatory landscapes, aiding efficient patient recruitment and guaranteeing that studies are performed ethically and in accordance with local laws. Stakeholders interested in advancing their medical device trials can engage with CROss to leverage these comprehensive services.
Top 10 Clinical Research Organizations to Know About
- bioaccess®: Focusing specifically on accelerated medical device research study services in Latin America, bioaccess® brings over 20 years of expertise in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF). Their specialized knowledge and flexibility ensure that U.S. medical device companies can navigate the complex regulatory landscape, including compliance with INVIMA, Colombia's National Food and Drug Surveillance Institute, recognized as a Level 4 health authority by PAHO/WHO.
With bioaccess®, partners gain a vetted CRO and consulting ally that is one of the best clinical research organizations dedicated to achieving successful clinical outcomes.
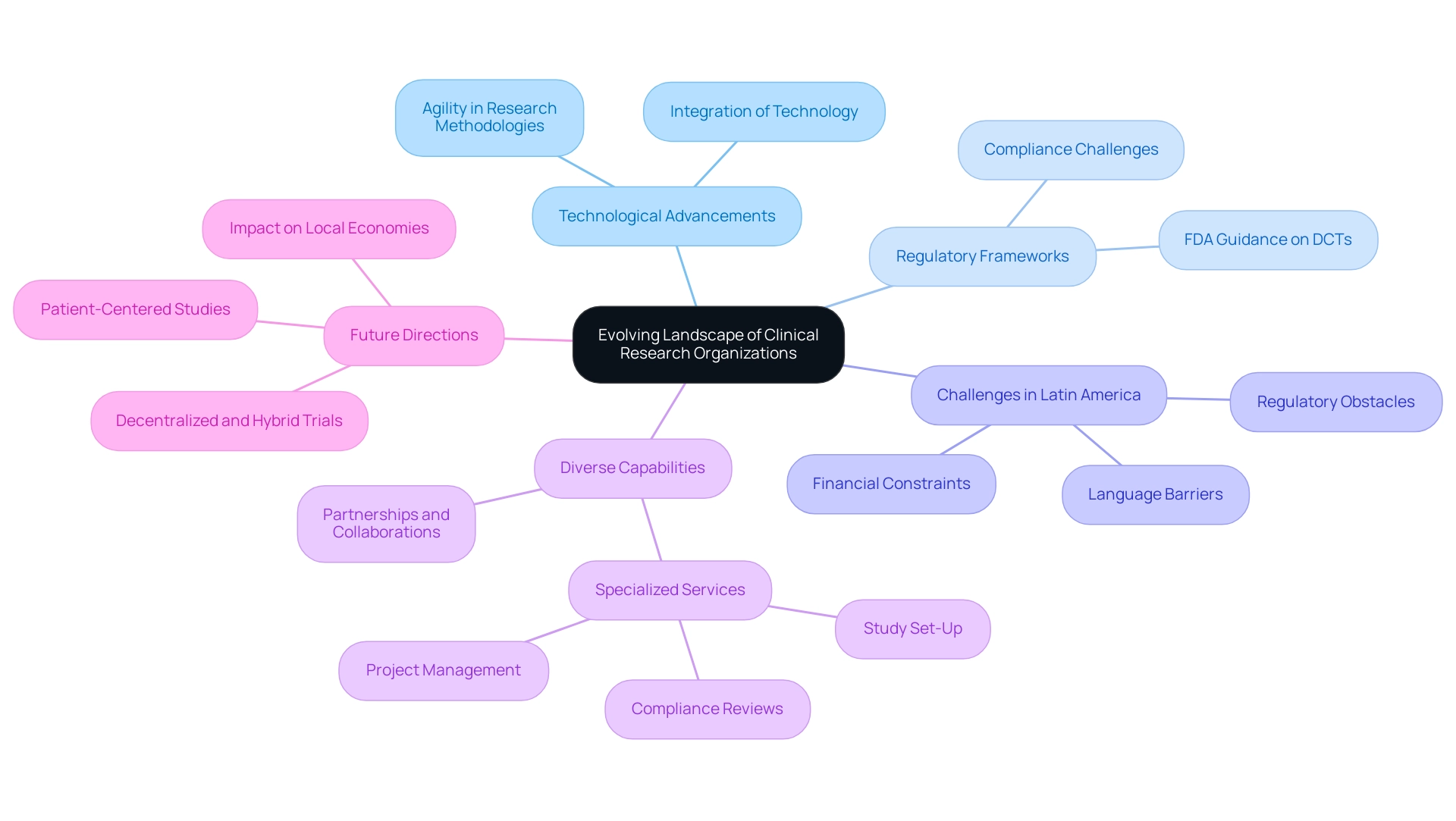
The Evolving Landscape of Clinical Research Organizations
The landscape of the best clinical research organizations is undergoing a significant transformation, largely driven by technological advancements, evolving regulatory frameworks, and a growing demand for specialized services. This is particularly relevant in Latin America, where Medtech companies encounter multifaceted challenges including regulatory obstacles, financial constraints, and language barriers. The particular service abilities of the best clinical research organizations, such as study set-up, compliance reviews, and project management, are essential for tackling these challenges.
As emphasized by industry leaders, the rise of the best clinical research organizations and academic study organizations has introduced a diverse array of players into the field, each bringing unique capabilities and perspectives that are essential for bridging these gaps. For example, partnerships such as the collaboration between Greenlight Guru and bioaccess™ are speeding up Medtech advancements and research in this area, as shown by PAVmed's first-in-human study in Colombia. This shift has led to an increased emphasis on agility and flexibility in research methodologies, prompting the emergence of the best clinical research organizations that focus on specific therapeutic areas or innovative approaches, thereby providing integrated solutions that encompass not only clinical trial management but also real-world evidence generation and patient engagement strategies.
Furthermore, as the industry navigates these transitions, there is a clear expectation for chief revenue officers to facilitate collaboration, which is key for driving global health improvement. Notably, Karishma Trikha, Chairperson of Executive Leadership, underscores this evolution by stating, 'The integration of technology and specialized focus will be crucial for Chief Risk Officers to remain competitive and effective in the coming years.' Furthermore, the sector encounters obstacles like minimal diversity efforts in research studies, emphasizing a domain requiring enhancement.
As we approach 2024, the emphasis on patient-centered studies and the implementation of decentralized and hybrid study designs—especially considering insights from the case analysis titled 'Current Opportunities and Outcomes in Rare Disease Clinical Trials,' which addresses the heightened interest in these methods due to the COVID-19 pandemic—will continue to influence the strategic directions of the best clinical research organizations, significantly impacting local economies through job creation and enhancements in healthcare.
The Importance of Diversity and Inclusion in Clinical Trials
Variety and inclusion in research studies are crucial for guaranteeing that findings are relevant across different segments of the population, especially for enhancing outcomes for minority communities experiencing healthcare disparities. In 2023, a noteworthy 69% of references concerning diversity in medical studies were attributed to US-based firms, highlighting a rising awareness of this essential issue. Rachael Fones, Director of Government & Public Affairs at IQVIA, powerfully articulates this necessity:
If we learn that one genetic marker impacts one race differently or more prevalently, that not only helps you develop your drug better, but it helps advance the science, advance the practice of medicine, and advance new therapies.
This insight highlights the profound implications of diversity in drug development and medical practices. As a result, the best clinical research organizations are increasingly acknowledging the significance of inclusive recruitment methods that involve diverse groups, thereby improving the validity of research outcomes. Fostering diversity enables CROss, one of the best clinical research organizations, to not only comply with regulatory requirements but also to uphold ethical research practices that promote health equity.
Furthermore, a recent call for inclusive preclinical data collection emphasizes the development of diverse cell lines and effective ancestry annotation policies, which are crucial for aligning preclinical safety data with the target patient population. This advocacy is directly connected to the broader aim of improving health equity in medical research. Overall, enhancing involvement from varied groups not only enriches the data gathered but also greatly influences research outcomes.
Leveraging Technology: AI and Machine Learning in Clinical Research
Artificial intelligence (AI) and machine learning are fundamentally transforming healthcare studies by enhancing efficiency, data analysis, and patient involvement. Significantly, Clinical Study Manager Dr. Sergio Alvarado is at the forefront of this evolution, leveraging innovative medical research to enhance studies in Latin America through bioaccess®, a leading Medtech organization focused on regulatory excellence and innovation. With a solid foundation as a general practitioner and experience in the medical affairs department at Novartis, Dr. Alvarado brings a wealth of knowledge to his role.
The best clinical research organizations (CROs) are increasingly utilizing these technologies to streamline patient recruitment, closely monitor study progress, and analyze extensive datasets with remarkable speed and precision. For instance, AI algorithms have shown an ability to lessen the burden of manual chart reviews by 40.5%, enabling researchers to identify appropriate candidates for trials through electronic health records more effectively, thus significantly shortening recruitment timelines. Notably, Brown et al. (2023) have automated endotracheal tube placement checks using deep learning, showcasing another critical application of AI in medical settings. Moreover, machine learning models are being utilized to predict patient outcomes and detect potential adverse events early, enabling proactive interventions. As these technologies advance, they hold the potential to further elevate the efficiency and quality of medical research, paving the way for swifter and more effective medical advancements.
Recent electronic searches carried out in various databases have uncovered pertinent studies concentrating on the use of AI technology in trial recruitment and retention, showcasing the increasing interest and advancement in this field. However, the insights from the systematic review titled Methodological Quality of AI Studies in Medical Imaging indicate an urgent need for higher quality, independent studies to accurately assess the effectiveness of these AI tools in medical imaging and beyond. This review revealed that many studies had serious or critical risks of bias, emphasizing the necessity for adherence to rigorous reporting guidelines.
As highlighted by Zihao Jiang, the senior author of a recent study, significant strides are being made in the conception and implementation of AI applications in clinical investigations, underscoring the importance of ongoing evaluation and the need for high-quality studies. Dr. Alvarado's commitment to continuous learning and effective communication further enhances his contributions to the field, ensuring that he remains at the cutting edge of medical research and technology.
Conclusion
The integral role of Clinical Research Organizations (CROs) in the modern clinical trial landscape cannot be overstated. They serve as critical facilitators, addressing the multifaceted challenges faced by medical device companies, particularly in regions like Latin America. Through their expertise in regulatory compliance, patient recruitment, and project management, CROs streamline the clinical trial process, ensuring efficiency and adherence to strict standards. The collaboration between entities such as bioaccess® and Caribbean Health Group exemplifies how CROs can position regions as emerging hubs for clinical research, significantly reducing recruitment times and improving retention rates.
The evolving landscape of clinical research is marked by technological advancements and a growing emphasis on diversity and inclusion. As CROs adopt innovative methodologies and leverage artificial intelligence, they enhance the quality and efficiency of trials, paving the way for more effective medical advancements. Moreover, the focus on inclusive recruitment strategies not only meets regulatory requirements but also enriches the data collected, ultimately contributing to better health outcomes across diverse populations.
As the demand for specialized services continues to rise, CROs are poised to play an even more pivotal role in advancing medical research. By fostering collaboration and embracing technological innovations, they are not only enhancing the efficiency of clinical trials but also driving global health improvements. The future of clinical research lies in the ability of CROs to adapt, innovate, and prioritize diversity, ensuring that research findings are relevant and beneficial for all segments of the population.
Frequently Asked Questions
What services do the best clinical research organizations (CROs) provide?
The best clinical research organizations contribute to the planning, execution, and management of studies by offering services such as feasibility and selection of study site and principal investigator, regulatory compliance, project management, and reporting.
How do CROs assist pharmaceutical, biotechnology, and medical device firms?
CROs enable a streamlined research process, ensuring studies are performed effectively and adhere to regulatory standards, which is crucial for medical device startups facing challenges like regulatory hurdles, competition, recruitment issues, and financial constraints.
Can you provide an example of a successful partnership involving a CRO?
An example is the partnership between bioaccess™ and Caribbean Health Group, which aims to establish Barranquilla as a leading location for medical studies in Latin America, supported by Colombia's Minister of Health. Additionally, GlobalCare Clinical Studies partnered with bioaccess™ to enhance clinical study ambulatory services in Colombia, achieving over a 50% reduction in recruitment time and a 95% retention rate.
What advantages do CROs offer in terms of patient recruitment and regulatory navigation?
The global reach of the best CROs allows access to diverse patient groups and facilitates navigation through complex regulatory landscapes, ensuring ethical conduct of studies in accordance with local laws and efficient patient recruitment.
What specific expertise does bioaccess® provide in medical device research?
Bioaccess® specializes in accelerated medical device research study services in Latin America, with over 20 years of experience managing Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies, while ensuring compliance with Colombia's INVIMA regulations.
How does bioaccess® support U.S. medical device companies?
Bioaccess® acts as a vetted CRO and consulting ally, helping U.S. medical device companies navigate the complex regulatory landscape in Colombia, recognized as a Level 4 health authority by PAHO/WHO, to achieve successful clinical outcomes.




