Overview
The article focuses on identifying the top ten biggest contract research organizations (CROs) that play a crucial role in the pharmaceutical and biotechnology sectors. It highlights their extensive services, industry influence, and ranking criteria based on revenue, client satisfaction, and global reach, showcasing how these organizations enhance drug development processes and contribute to healthcare improvements.
Introduction
In the ever-evolving landscape of medical research, Contract Research Organizations (CROs) have emerged as vital players, bridging the gap between innovative therapies and their successful market entry. These organizations offer a spectrum of specialized services that streamline clinical trials, from site feasibility and patient recruitment to regulatory compliance and data management.
As the demand for efficient and effective clinical research intensifies, particularly in regions like Colombia—which boasts unique advantages for first-in-human trials—the role of CROs becomes increasingly significant. This article delves into the essential functions of CROs, evaluates the criteria for ranking the top organizations in the field, and highlights key players such as:
- IQVIA
- Parexel
- Medpace
showcasing their contributions to advancing healthcare solutions and improving patient outcomes across the globe.
1. Understanding Contract Research Organizations (CROs): An Overview
The biggest contract research organizations (CROs) act as essential collaborators in the pharmaceutical, biotechnology, and medical device industries, providing specialized outsourced services that include a thorough process for progressing medical device evaluations. They play an essential role in:
- Site feasibility
- Investigator selection
- Study set-up
- Start-up approvals
- Regulatory compliance
- Project management
- Monitoring
This ensures adherence to regulatory standards and facilitates the development of innovative therapies. Among the extensive services they provide are:
- Study design
- Patient recruitment
- Data management
- Regulatory submissions
- Ongoing monitoring
Each is vital for the successful execution of research projects.
Significantly, Colombia offers competitive benefits for first-in-human studies, including:
- Cost efficiency
- Regulatory speed
- High-quality healthcare
- Patient recruitment
- R&D tax incentives
This environment is further enhanced by the collaboration between bioaccess™ and Caribbean Health Group, which aims to position Barranquilla as a leading destination for medical trials in Latin America, supported by Colombia's Minister of Health. This governmental support highlights the dedication to making Barranquilla an appealing center for clinical studies.
By leveraging the expertise of the biggest contract research organizations, sponsors can focus on their core competencies while benefiting from enhanced efficiency and reduced costs in the study process. As David Maislin aptly states,
Their expertise and commitment to patient-focused studies ensure they will continue delivering impactful healthcare solutions.
Notably, PSI, a fast-growing CRO, received the CRO Leadership Awards for Expertise, Quality, and Reliability in June 2023, underscoring its commitment to excellence in the industry.
The global influence of the biggest contract research organizations, such as IQVIA, Thermo Fisher, and Parexel International, enhances their importance, enabling them to execute studies across various populations and navigate international regulations. This capability not only provides a competitive edge but also aligns with the needs of a rapidly evolving healthcare landscape, where an estimated 20 million new cancer cases were diagnosed in 2022. The capacity to reach diverse patient groups guarantees that investigations are performed ethically and with cultural awareness, thus improving the overall quality and significance of medical inquiries.
2. Criteria for Ranking the Top Contract Research Organizations
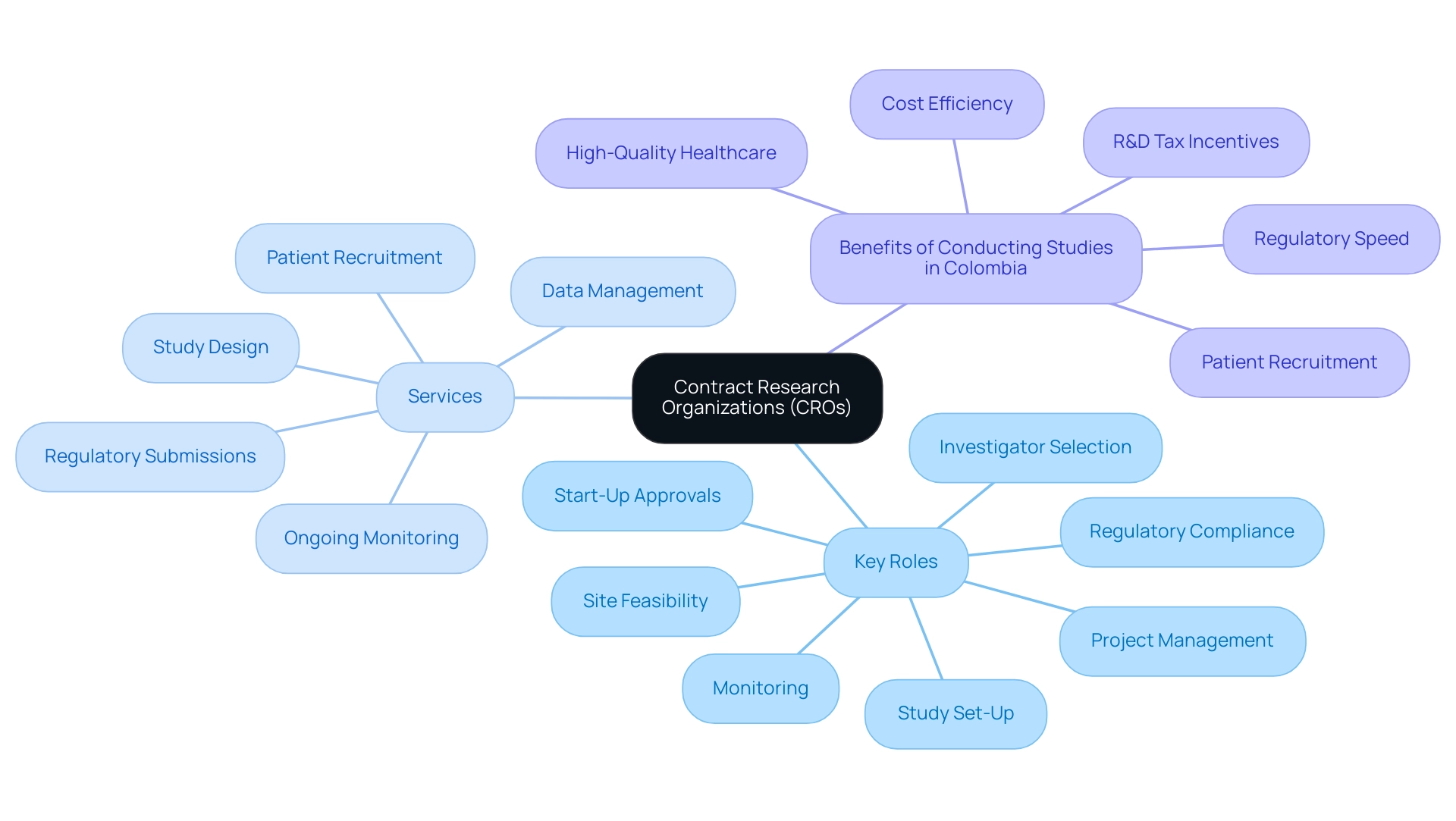
The ranking of the biggest contract research organizations (CROs) is determined by a comprehensive evaluation of multiple criteria, including:
- Revenue performance
- Market share
- Global reach
Client satisfaction is crucial, as it reflects the effectiveness of the CRO in meeting the needs of its partners. As IBISWorld notes, client satisfaction is paramount as it directly influences the rankings of CROs and their reputation in the industry.
Furthermore, organizations are evaluated based on their service offerings, particularly in comprehensive trial management services such as:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
In 2024, for instance, PSI has been recognized for its exceptional performance, receiving CRO Leadership Awards for six consecutive years, highlighting its expertise, quality, and reliability. Additionally, TFS received a Silver Rating from EcoVadis in 2024, placing it in the top 15% for sustainability excellence, which underscores the importance of sustainability in the evaluation criteria.
Adaptability to evolving industry demands plays a vital role in these rankings, ensuring that the biggest contract research organizations not only lead in size but also significantly contribute to advancing clinical studies, enhancing patient outcomes, and positively impacting local economies through job creation and economic growth. The biggest contract research organizations in the CRO industry offer essential study services on a contract basis to the pharmaceutical and biotechnology sectors, playing a vital role in supporting drug and medical device development through various study services. This encompasses detailed procedures such as:
- The review and feedback on research documents to comply with country requirements
- Obtaining ethics committee and health ministry approvals for trial setup
- Ongoing monitoring and reporting of research status and adverse events
This multifaceted approach to evaluation underscores the importance of both quantitative and qualitative factors in determining the top performers in the CRO landscape.
3. IQVIA: Leading the Charge in Clinical Research
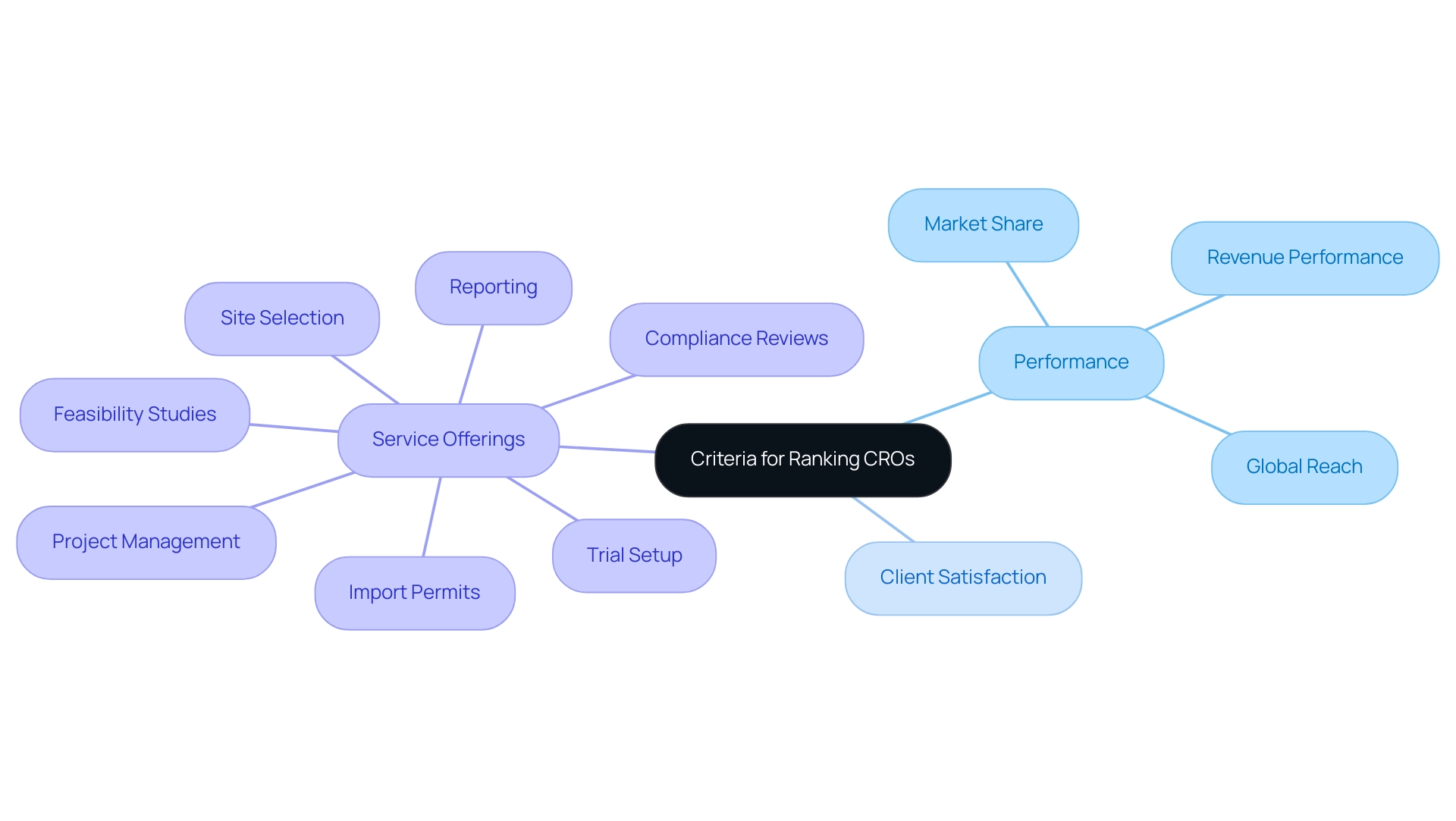
As one of the biggest contract research organizations in the healthcare field, IQVIA utilizes its vast data analysis and advanced technology to provide thorough solutions for its clients. Our service capabilities include:
- Feasibility assessments
- Selection of research locations and principal investigators
- Compliance reviews—including feedback on documents to ensure adherence to country requirements
- Trial setup involving ethics committee and health ministry approvals
- Project management
This ensures that all phases of medical development are supported.
As one of the biggest contract research organizations, this organization seamlessly transitions from preclinical studies to post-market surveillance, with methodologies that incorporate real-world evidence alongside advanced analytics, allowing for enhanced decision-making processes that ultimately lead to improved patient outcomes. In 2023, there were 24 first-in-class launches in the U.S., highlighting the dynamic nature of the research environment and IQVIA's pivotal role within it. With North America accounting for more than 33% of the worldwide research software market, propelled by supportive regulations and governmental assistance, IQVIA, one of the biggest contract research organizations, plays a key role in this expansion, supported by its dedication to quality and adherence.
Moreover, the influence of our research efforts reaches local economies, promoting job creation, healthcare enhancement, and international cooperation. As Marlene Greenfield, Vice President at Hearst Magazines, aptly puts it,
Statista has been my savior on several occasions. The site is easy to maneuver and the data is in a format that can go right into a report or presentation,
highlighting the importance of accessible data in the industry.
Furthermore, the success of one of the biggest contract research organizations, IQVIA, is reflected in a notable CAGR increase in the U.S. market, projected to reach 2-5% through 2028, driven by heightened patient engagement and the adoption of high-value therapies. The insights from the case examination titled 'Geographical Market Analysis' further highlight that the area's current infrastructure and governmental assistance greatly aid in the expansion of medical investigations and the software market for healthcare experiments, emphasizing IQVIA's essential role in influencing the future of medical exploration.
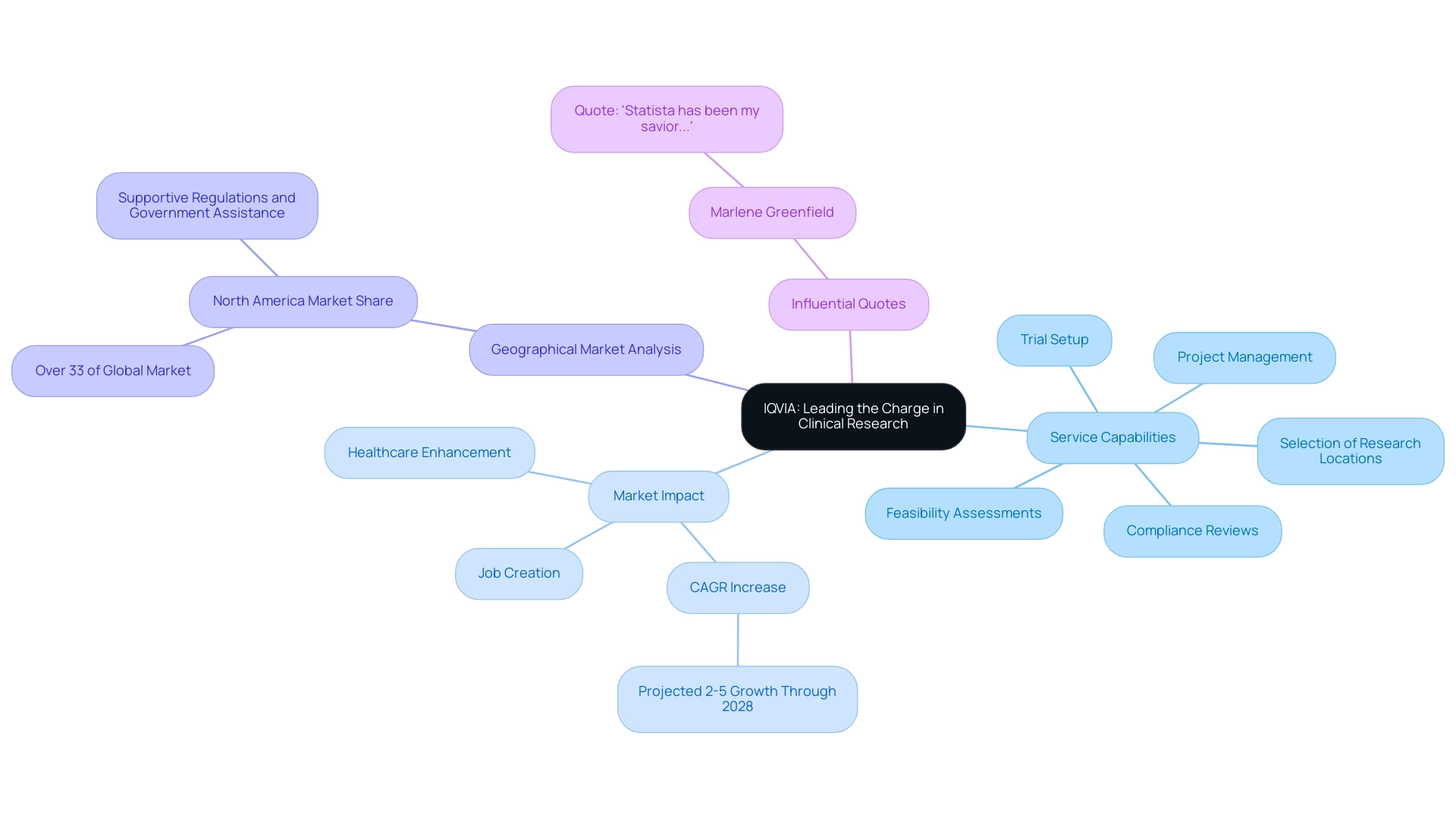
4. Parexel: Innovating Clinical Development Solutions
Parexel stands out in the research landscape for its innovative development solutions designed to enhance the research process. With the market size expected to reach USD 84.7 billion in 2024, Parexel's extensive array of services—including feasibility studies, site selection, compliance reviews, setup, import permits, project management, and comprehensive reporting on study status, inventory, and adverse events—prioritizes patient-centric approaches and adaptive study designs. This emphasis has established the organization as a leader in the industry, setting new benchmarks for excellence in care.
Recent advancements in study management highlight Parexel's dedication to utilizing technology and data analytics, which not only enhance study efficiency but also speed up the introduction of new therapies to market. For example, Parexel's innovative solutions have been crucial in enhancing patient engagement and optimizing study outcomes. In 2024, these strategies are anticipated to transform development metrics, with Parexel spearheading the movement towards more effective and patient-centered studies.
Experts within Parexel affirm that by adopting such approaches, the industry can foster a more collaborative environment, ultimately benefiting both patients and stakeholders alike. Additionally, as noted by Florence Mowlem, PhD, Vice President of Science for ObvioHealth:
- "I hope this can be a turning point for the industry with regard to comparability testing. We can stop having [comparability] conversations so frequently, and instead we can start talking about optimizing our electronic measures for all individuals."
Furthermore, PSI, a fast-growing CRO specializing in oncology, hematology, and infectious diseases, has demonstrated success in the industry, receiving CRO Leadership Awards for Expertise, Quality, and Reliability for the sixth consecutive year. This highlights the competitive landscape in which Parexel operates, as it is recognized among the biggest contract research organizations, and underscores its commitment to excellence, particularly in driving global health improvement through international collaboration and innovation in medtech.
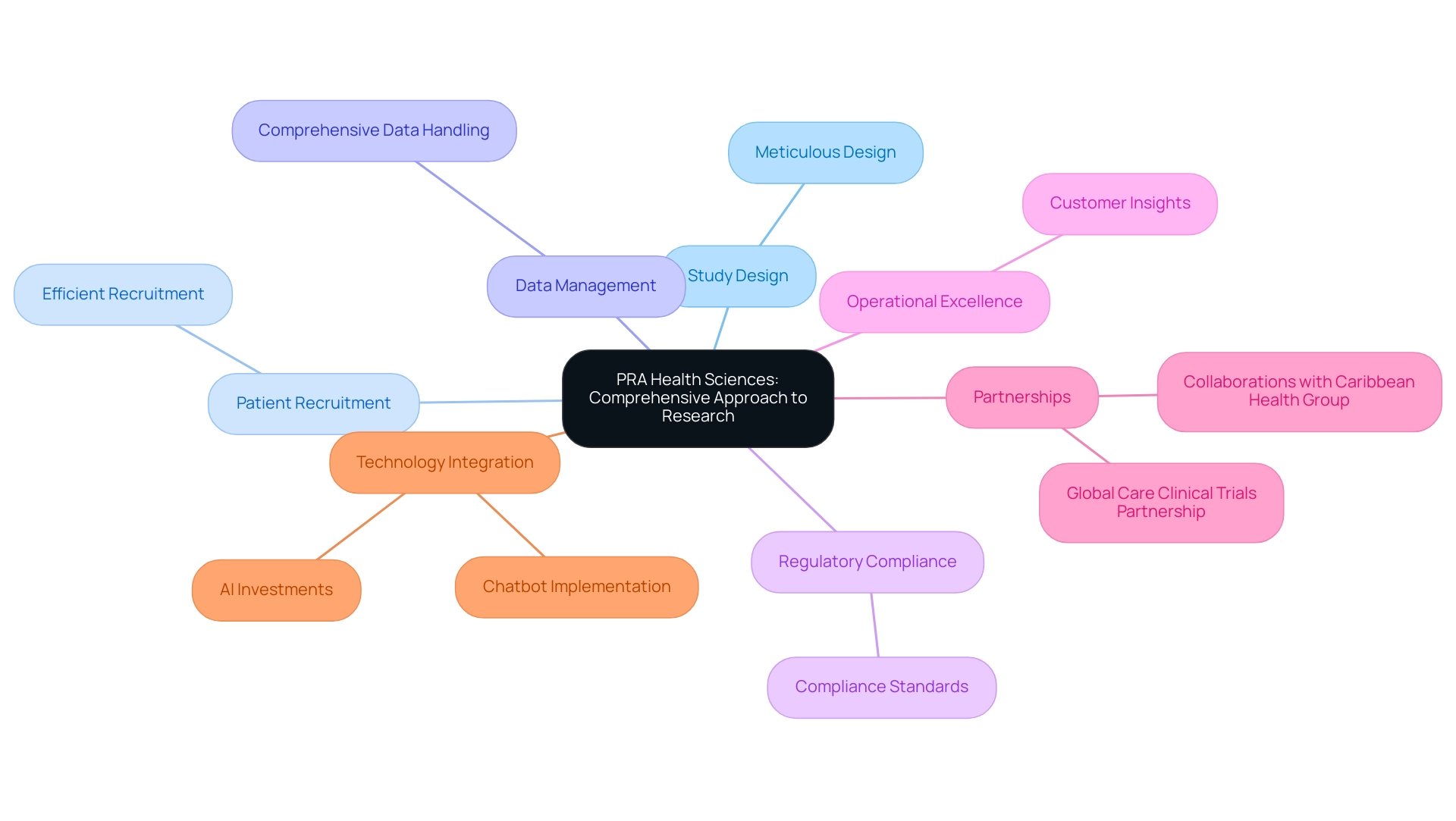
5. PRA Health Sciences: A Comprehensive Approach to Research
PRA Health Sciences distinguishes itself in the field of medical studies for its comprehensive approach, offering a wide range of services that cover every aspect of trials. Their offerings range from meticulous study design and efficient patient recruitment to comprehensive data management and stringent regulatory compliance. This comprehensive assistance is crucial for clients maneuvering through the intricacies of medical studies.
Pra's unwavering dedication to quality and operational excellence has established them as a trusted ally for pharmaceutical and biotech firms. Notably, industry leaders emphasize that operational excellence in the biggest contract research organizations (CROs) is critical for driving successful project outcomes. As one industry leader stated,
'There is only one boss.
The customer. And he can fire everybody in the company from the chairman on down, simply by spending his money somewhere else.'
Furthermore, Pra's commitment to fostering innovation and collaboration not only enhances their operational efficiency but also significantly contributes to delivering positive results for clients and improving patient care.
In Latin America, bioaccess™ has risen as a prominent contract organization, particularly partnering with Caribbean Health Group to establish Barranquilla as a top location for studies, backed by Colombia's Minister of Health. Their partnership with Global Care Clinical Trials has yielded remarkable results, achieving over a 50% reduction in recruitment time and an impressive 95% retention rate. Input from customers consistently highlights Pra's efficiency, showcasing their skill in overseeing successful studies and attaining significant progress in medical science.
As the industry evolves, with 40% of companies planning to invest in chatbots for their call centers and 70% of C-level support executives planning to invest in AI in 2024, Pra's integration of cutting-edge technologies into their services positions them at the forefront of the industry. Additionally, users can manage their cookie preferences at any time to enhance their website experience, ensuring compliance and transparency in their interactions.
6. ICON plc: Global Expertise in Clinical Trials
As one of the biggest contract research organizations (CROs), ICON plc boasts extensive expertise in managing clinical studies across various therapeutic areas. Their extensive service capabilities encompass:
- Feasibility and selection of research locations
- Principal investigator (PI) selection
- Compliance reviews of research documents
- Setup
- Import permits
- Nationalization of investigational devices
- Ethics committee approvals
With a global footprint spanning over 40 countries, ICON, recognized as one of the biggest contract research organizations, provides clients invaluable access to diverse patient populations and regulatory environments, driving efficient trial execution and high-quality results.
This strategic positioning is further solidified by their commitment to project management and thorough reporting on study status, including serious and non-serious adverse events. In light of industry growth, as shown by Medpace's plans to create 1,500 jobs and invest $150 million in expansion, ICON, recognized as one of the biggest contract research organizations, continues to demonstrate its commitment to innovation and excellence in medical studies. Their patient recruitment strategies and site management abilities are crucial in fostering study success, contributing not only to healthcare improvement but also to job creation and economic growth in local economies.
As they revise their research studies for 2024, ICON demonstrates a commitment to remaining at the forefront in the evolving field of medical research, highlighting their capacity to adjust and succeed in a competitive market. This aligns with the industry's sentiment that growth and innovation are essential for the advancement of medical science and improving patient care.
![]()
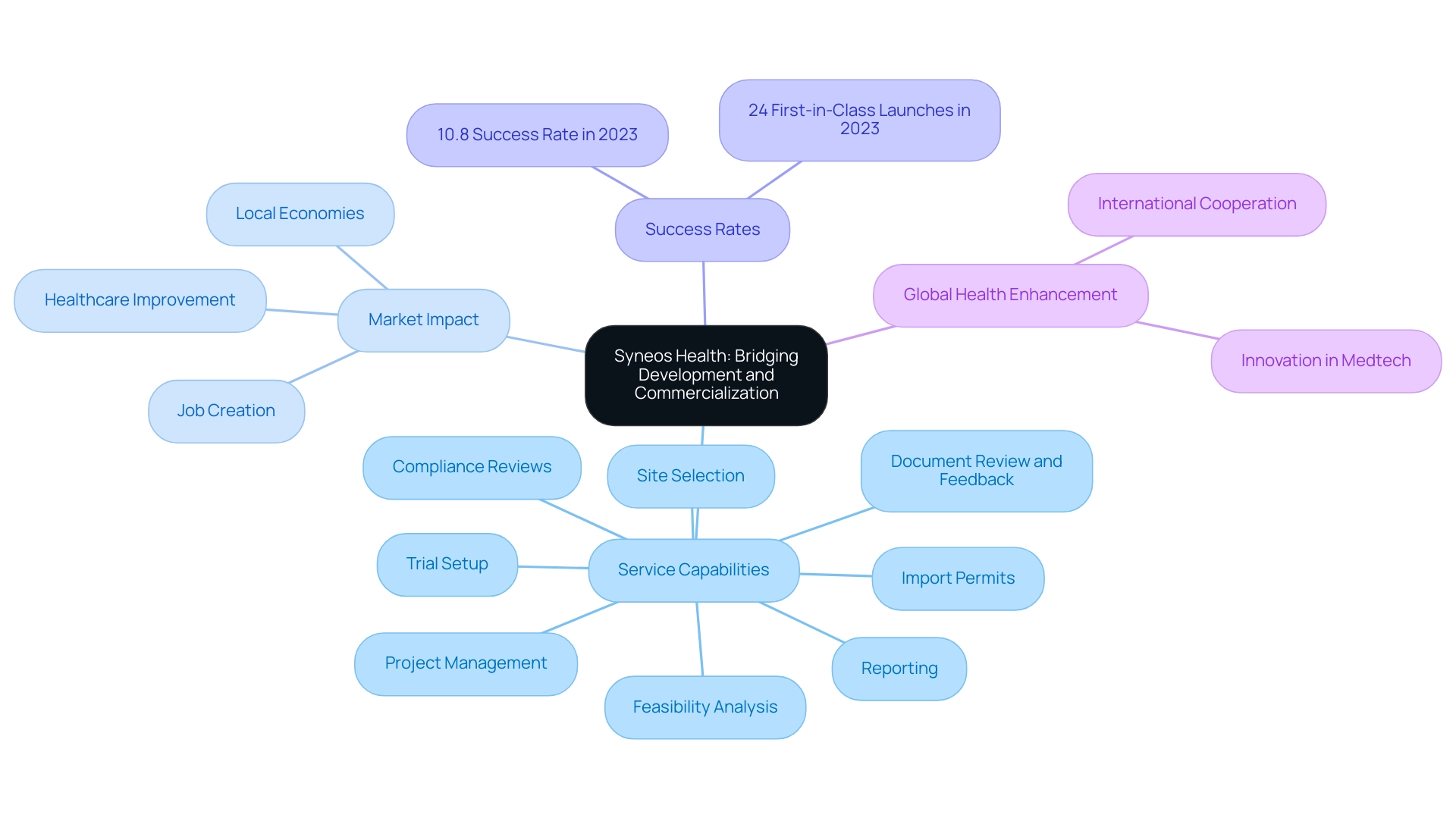
7. Syneos Health: Bridging the Gap Between Development and Commercialization
Syneos Health distinguishes itself in the industry due to its integrated model that effectively connects development and commercialization. Their comprehensive service capabilities encompass:
- Feasibility analysis
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
- Review and feedback on documents to adhere to country requirements
This empowers clients to navigate the complexities associated with bringing innovative therapies to market.
By seamlessly merging healthcare and commercial services, Syneos Health improves patient engagement, which is essential for the overall success rates of new product launches. The 2023 landscape saw 24 first-in-class launches in the U.S., underscoring the importance of robust market access strategies. As Murray Aitken noted, 'The composite success rate for the pipeline jumped in 2023 to 10.8% across all therapy areas after falling to a 10-year low in 2022, driven by increases in Phase I, Phase III and regulatory success.'
This statistic highlights the improving industry performance, which Syneos Health is well-positioned to leverage. Furthermore, their emphasis on advancing global health enhancement through international cooperation and innovation in Medtech corresponds with the wider influence of clinical research on local economies, including job creation, economic growth, and healthcare improvement. Syneos Health's services contribute to these outcomes by fostering local partnerships and supporting workforce development initiatives.
The case study of HCG illustrates how Syneos Health's integrated approach accelerates the impact of scientific innovations on real-world healthcare, emphasizing the importance of streaming campaigns for pharma brands to enhance awareness and sales. Syneos Health leaders stress that successful commercialization relies on collaboration and innovation, further reinforcing the organization’s position as a crucial ally for companies seeking to enhance both development and commercialization efforts. Their proactive approach not only fosters a successful trajectory for their clients but also aligns with the industry's evolving demands.
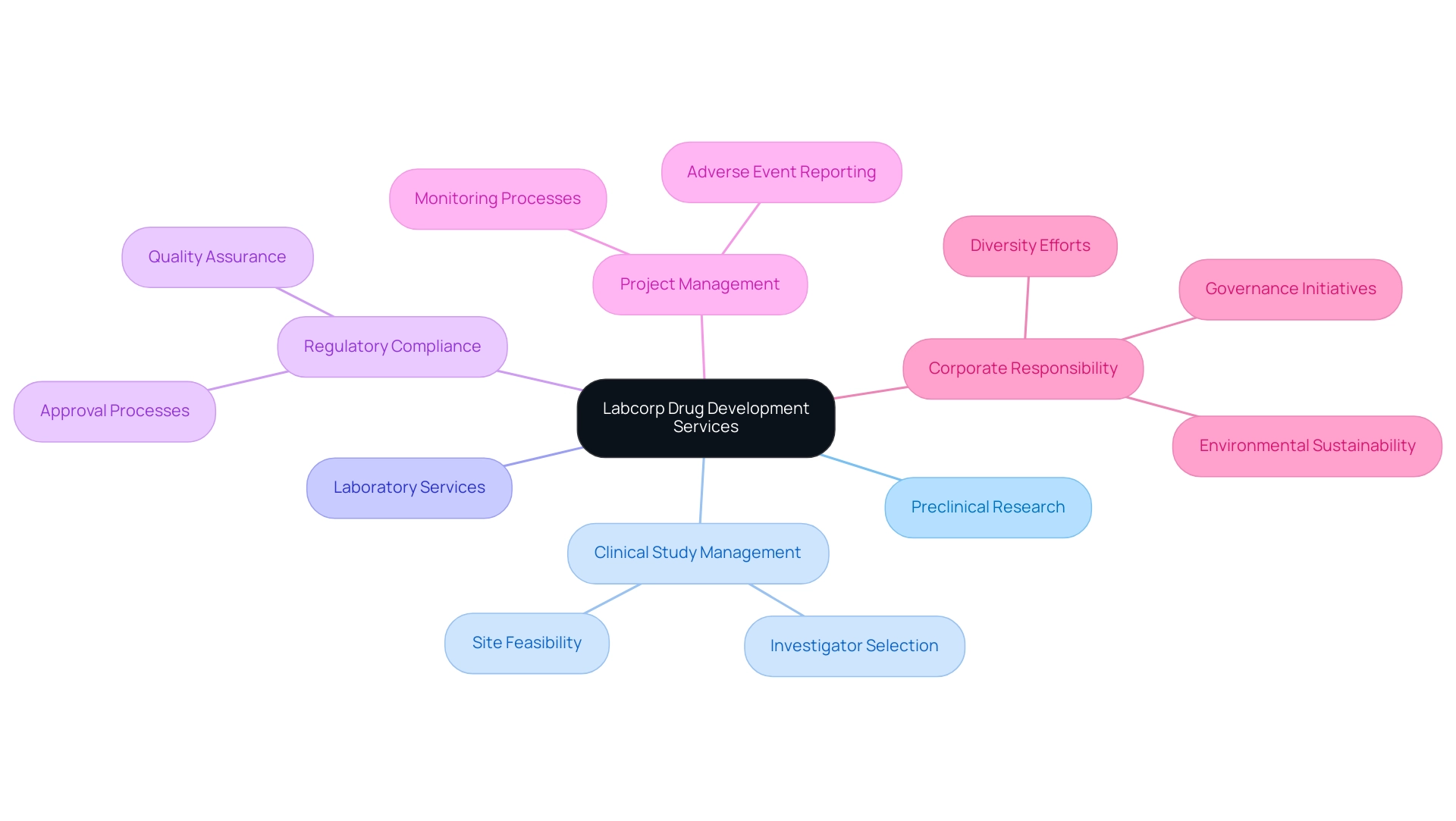
8. Labcorp Drug Development: Comprehensive Drug Development Services
Labcorp Drug Development stands out as one of the biggest contract research organizations (CROs), recognized for its extensive range of drug development services that include preclinical research, clinical study management, and laboratory services. Their expertise in site feasibility and investigator selection ensures that studies are conducted efficiently and in compliance with regulatory requirements. Additionally, Labcorp excels in study set-up, start-up, and obtaining necessary approvals from ethics committees and health ministries, which are critical for advancing medical device studies.
This extensive array of capabilities empowers clients to effectively streamline their drug development processes, significantly reducing time to market. Recent advancements within Labcorp have been crucial; for instance, the company's research management services have been improved, reflecting their commitment to operational excellence and regulatory compliance. As noted by Leslie Rivera Rosado, a supervisory interdisciplinary scientist at the FDA, 'The FDA has observed a rise in complete response letters (CRLs) issued to sponsors of biologics license applications (BLAs) over the last decade, with facility deficiencies being the most common reason cited.'
This highlights the critical importance of quality assurance in Labcorp's operations. Furthermore, in relation to Colombia's competitive advantages, Labcorp, one of the biggest contract research organizations, utilizes cost efficiency, regulatory speed, and high-quality healthcare, positioning itself advantageously for first-in-human studies. By harnessing their global network and deep expertise, Labcorp not only supports clients in advancing their drug development programs but also aims to enhance patient outcomes.
Their project management and monitoring processes ensure that study status and inventory are meticulously reported, including serious and non-serious adverse events. Their 2023 Corporate Responsibility Report explicitly details their commitment to corporate responsibility, showcasing initiatives in governance, diversity, and environmental sustainability that align with their corporate values. Through these efforts, Labcorp continues to play a transformative role in the pharmaceutical landscape, driving innovation and success in research studies.
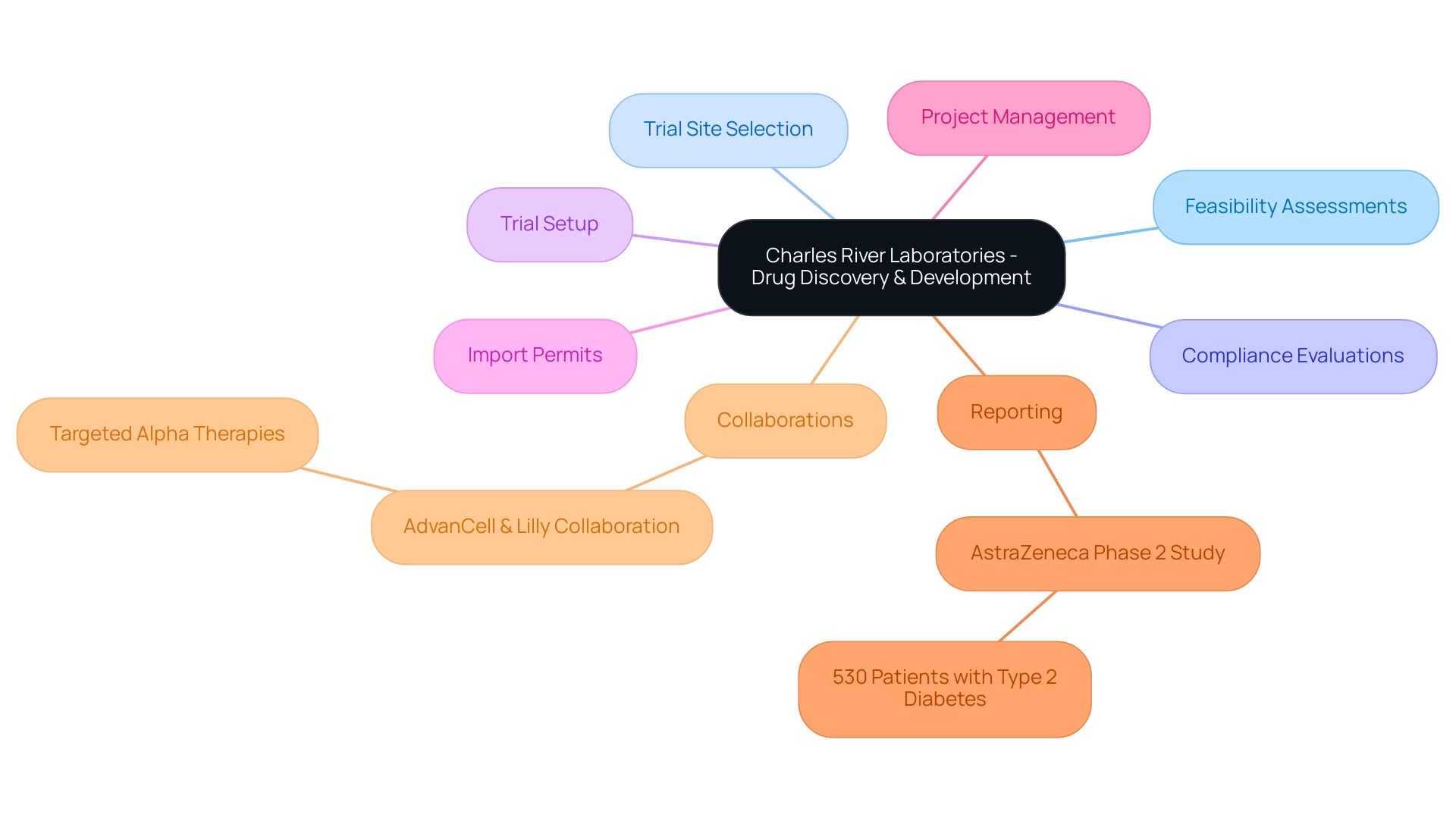
9. Charles River Laboratories: Advancing Drug Discovery and Development
Charles River Laboratories is a leader among the biggest contract research organizations, specializing in the essential area of drug discovery and development. Their extensive clinical trial management services encompass:
- Feasibility assessments
- Selection of trial sites and lead investigators
- Compliance evaluations of documentation to fulfill country requirements
- Trial setup
- Import permits
- Project management
- Reporting on trial status, inventory, and adverse events
This robust suite of services is designed to support the pharmaceutical and biotechnology sectors in making informed decisions during the early stages of drug development.
A notable example of their impact is seen in AstraZeneca's phase 2 study involving 530 patients with type 2 diabetes, which underscores the significance of Charles River's services in advancing drug development. Through their innovative methodologies and international collaborations, Charles River empowers clients to optimize strategies and enhance the likelihood of successful outcomes, driving global health improvement in the Medtech sector. As Charlie Sternberg notes, 'The lab in California is fully operational and has already released its first Active Pharmaceutical Ingredient,' reflecting their commitment to excellence and innovation.
Recent statistics indicate a marked increase in success rates for drug discovery initiatives associated with Charles River, one of the biggest contract research organizations, providing further evidence of their pivotal role in the industry. Moreover, significant collaborations, like the one with AdvanCell and Lilly, demonstrate how they utilize advanced production technologies and drug candidate programs, improving their clients' capabilities and strengthening their reputation as a reliable partner in advancing drug development initiatives globally.
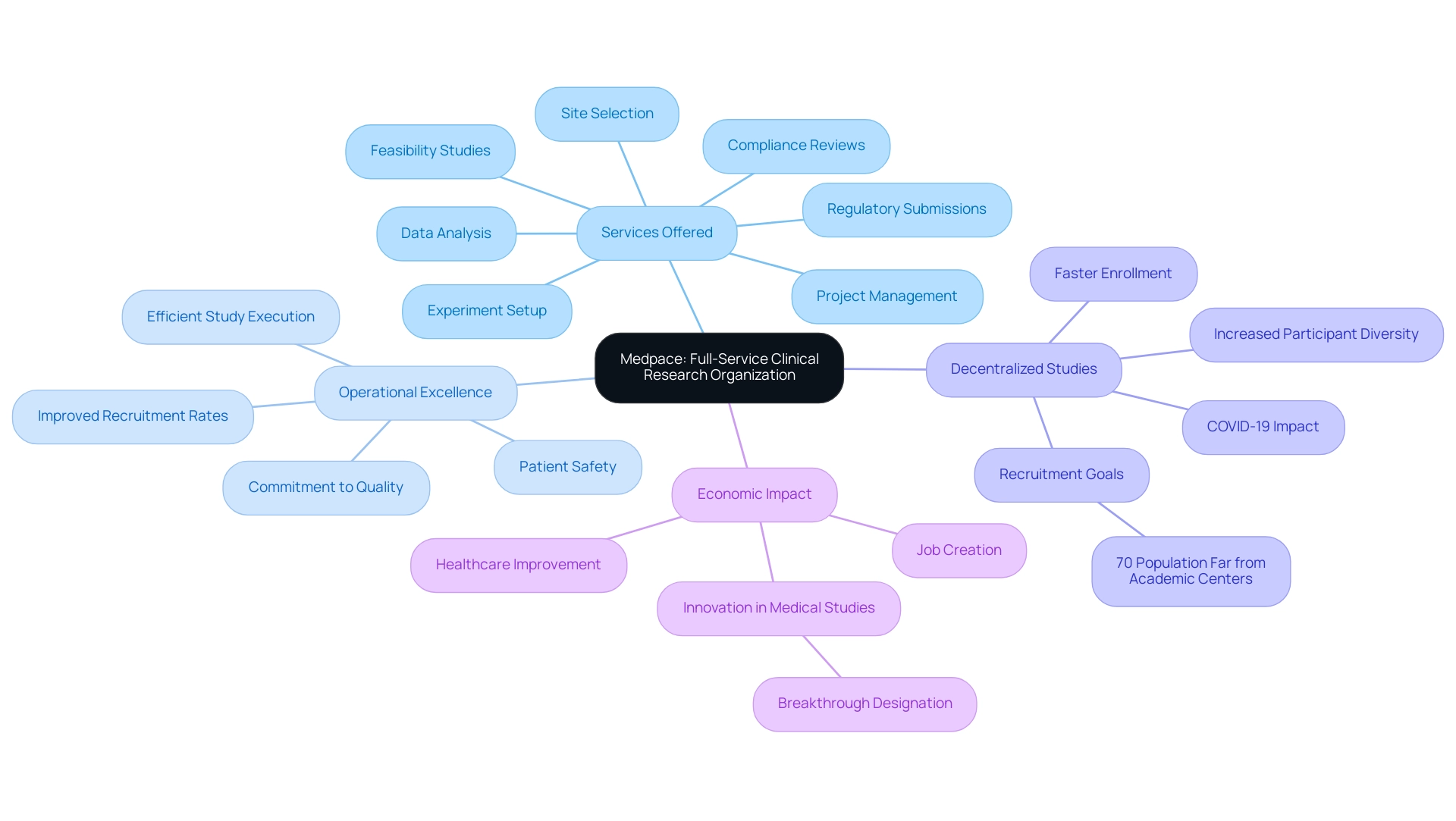
10. Medpace: A Full-Service Clinical Research Organization
Medpace distinguishes itself as one of the biggest contract research organizations by providing a broad range of services aimed at supporting every stage of clinical studies. Their expertise ranges from feasibility studies and site selection to experiment setup, project management, compliance reviews, data analysis, and regulatory submissions, enabling them to offer clients comprehensive and integrated support throughout the research process. Medpace places a strong emphasis on operational excellence, which is reflected in their efficient study execution and commitment to quality and patient safety.
This commitment is especially pertinent in the context of decentralized studies, which have gained traction due to the COVID-19 pandemic. As emphasized in a recent case analysis, decentralized trials can enable faster enrollment, increase participant diversity, and help research achieve recruitment goals, especially for the 70% of the population living far from academic medical centers. Medpace’s operational excellence metrics underscore the effectiveness of their methodologies, allowing for significant improvements in recruitment rates.
Furthermore, the economic impacts of Medtech research studies are notable, contributing to job creation and healthcare improvement in local economies. As Dave Marver, CEO of ONWARD Medical, mentioned at the 23rd Annual Needham Virtual Healthcare Conference, 'This makes us the FIRST company in the world to receive breakthrough designation for a BCI paired with therapeutic stimulation,' highlighting the significance of innovation and quality in medical studies. Medpace’s strategic approach establishes them among the biggest contract research organizations, making them an invaluable partner for organizations aiming to advance their clinical research initiatives while maintaining the highest standards of excellence.
Conclusion
The vital role of Contract Research Organizations (CROs) in the realm of clinical research cannot be overstated. These organizations are instrumental in facilitating the efficient progression of innovative therapies from concept to market, ensuring that clinical trials are conducted with the utmost quality and compliance. By leveraging their extensive expertise in areas such as:
- Site feasibility
- Patient recruitment
- Regulatory submissions
- Data management
CROs not only help streamline the research process but also significantly enhance patient outcomes.
As highlighted in this article, several top-tier CROs, including IQVIA, Parexel, and Medpace, exemplify the standards of excellence that drive the industry forward. Their commitment to innovation, patient-centric approaches, and operational excellence underscores their importance in navigating the complexities of clinical trials. The competitive advantages offered by regions like Colombia further illustrate the global landscape of clinical research, showcasing how local collaboration and government support can create optimal environments for conducting first-in-human trials.
Ultimately, the continued evolution and adaptation of CROs to meet the demands of a rapidly changing healthcare environment are crucial for advancing medical science. As these organizations enhance their capabilities and expand their reach, they will play an increasingly pivotal role in bringing life-saving therapies to patients around the world. The future of clinical research is bright, and CROs stand at the forefront, ready to lead the charge in delivering impactful healthcare solutions.
Frequently Asked Questions
What role do contract research organizations (CROs) play in the pharmaceutical and biotechnology industries?
CROs act as essential collaborators by providing specialized outsourced services that include site feasibility, investigator selection, study set-up, start-up approvals, regulatory compliance, project management, and monitoring, which ensures adherence to regulatory standards and facilitates the development of innovative therapies.
What specific services do CROs offer?
CROs provide a variety of services, including study design, patient recruitment, data management, regulatory submissions, and ongoing monitoring, all of which are vital for the successful execution of research projects.
What advantages does Colombia offer for first-in-human studies?
Colombia offers competitive benefits such as cost efficiency, regulatory speed, high-quality healthcare, effective patient recruitment, and R&D tax incentives, making it an attractive location for medical trials.
How is Barranquilla positioned in the context of clinical studies in Latin America?
Barranquilla is being positioned as a leading destination for medical trials in Latin America through collaboration between bioaccess™ and Caribbean Health Group, supported by Colombia's Minister of Health, highlighting governmental dedication to enhancing its appeal for clinical studies.
How do CROs help sponsors in the research process?
By leveraging the expertise of CROs, sponsors can focus on their core competencies while benefiting from enhanced efficiency and reduced costs throughout the study process.
What recognition has PSI received in the CRO industry?
PSI has been recognized with the CRO Leadership Awards for Expertise, Quality, and Reliability for six consecutive years, underscoring its commitment to excellence in the industry.
What factors determine the ranking of the biggest CROs?
The ranking is based on revenue performance, market share, global reach, client satisfaction, and the effectiveness of service offerings, particularly in comprehensive trial management services.
What specific services are included in comprehensive trial management by CROs?
Services include feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting.
How does adaptability to industry demands affect CRO rankings?
Adaptability ensures that CROs not only lead in size but also significantly contribute to advancing clinical studies, enhancing patient outcomes, and positively impacting local economies.
What is IQVIA's role as a CRO?
IQVIA utilizes data analysis and advanced technology to provide thorough solutions, including feasibility assessments, site selection, compliance reviews, trial setup, and project management, supporting all phases of medical development.
How does IQVIA contribute to the healthcare market?
IQVIA plays a key role in the expansion of the research software market and medical investigations, supported by its dedication to quality and adherence to regulations, promoting job creation and healthcare enhancement.
What is the projected growth for the U.S. market in the CRO sector?
The U.S. market is projected to experience a CAGR increase of 2-5% through 2028, driven by heightened patient engagement and the adoption of high-value therapies.




