Overview
The article focuses on the top 10 clinical research organizations (CROs) globally, which play a crucial role in facilitating the development of new therapies and ensuring compliance with regulatory standards in clinical research. It highlights their diverse offerings, such as feasibility studies and project management, and underscores their significance in addressing challenges faced by medical device startups, as well as their innovative approaches that enhance patient engagement and improve study outcomes.
Introduction
In the dynamic world of clinical research, Clinical Research Organizations (CROs) are emerging as indispensable allies in the quest for innovative therapies and effective drug development. As the global landscape evolves, these organizations are not only streamlining clinical trials but also addressing the myriad challenges faced by medical device startups and pharmaceutical companies.
From regulatory compliance to patient engagement and innovative methodologies, CROs offer a comprehensive suite of services that enhance trial efficiency and success rates. With the increasing complexity of clinical trials and the growing demand for transparency and ethical practices, understanding the pivotal role of CROs becomes essential for stakeholders aiming to navigate this intricate ecosystem successfully.
This article delves into the key functions of CROs, evaluates the criteria for selecting top organizations, and highlights industry leaders who are setting new standards in clinical research.
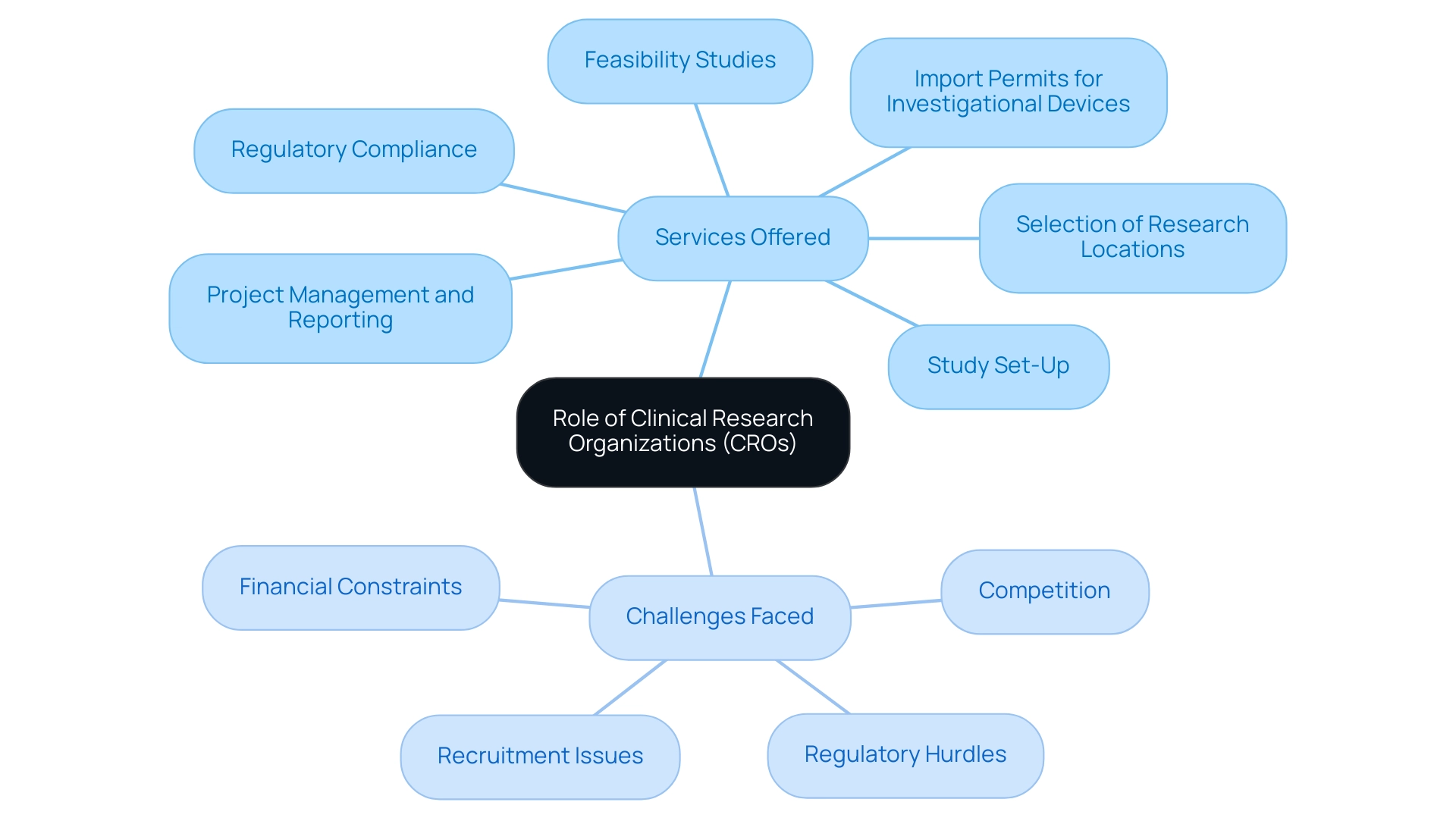
1. Understanding the Role of Clinical Research Organizations (CROs)
Within the healthcare ecosystem, the top 10 clinical research organizations in the world act as essential entities, providing a thorough array of specialized offerings that support the planning, execution, and management of research studies. These offerings encompass:
- Feasibility studies
- Selection of research locations and lead investigators
- Regulatory compliance
- Study set-up
- Import permits for investigational devices
- Rigorous project management and reporting
As the landscape of drug development evolves, with projections indicating that the global R&D pipeline will encompass approximately 22,825 drugs by 2024, the significance of the top 10 clinical research organizations in the world becomes increasingly apparent.
They are instrumental in facilitating the development of new therapies, ensuring adherence to regulatory standards, and providing strategic guidance throughout the research process, which positions them among the top 10 clinical research organizations in the world. The recent collaboration between GlobalCare Clinical Trials and bioaccess™ exemplifies this, showcasing an impressive reduction of over 50% in subject recruitment duration and a retention rate exceeding 95% as they expand ambulatory services in Colombia. Colombia itself provides competitive benefits for first-in-human studies, including cost efficiency, regulatory speed, and high-quality healthcare, which are essential for attracting medical device startups.
However, these startups often face challenges such as:
- Regulatory hurdles
- Competition
- Recruitment issues
- Financial constraints
In this context, CROs play an essential role in addressing these challenges while navigating the complexities of ethical concerns in medical research. Moreover, the partnership between IDx Technologies and bioaccess™ for data-licensing in Latin America emphasizes the increasing incorporation of AI in medical studies, especially in improving disease identification in ophthalmology.
As evidenced by a cost analysis of clinical studies in Switzerland, which revealed an average expenditure of approximately 72,000 USD per study, the need for transparency in clinical study costs is paramount, underscoring the essential role of CROs in financial management and ethical research practices. To discover more about how our offerings can enhance your medical device experiments, SCHEDULE A MEETING today.
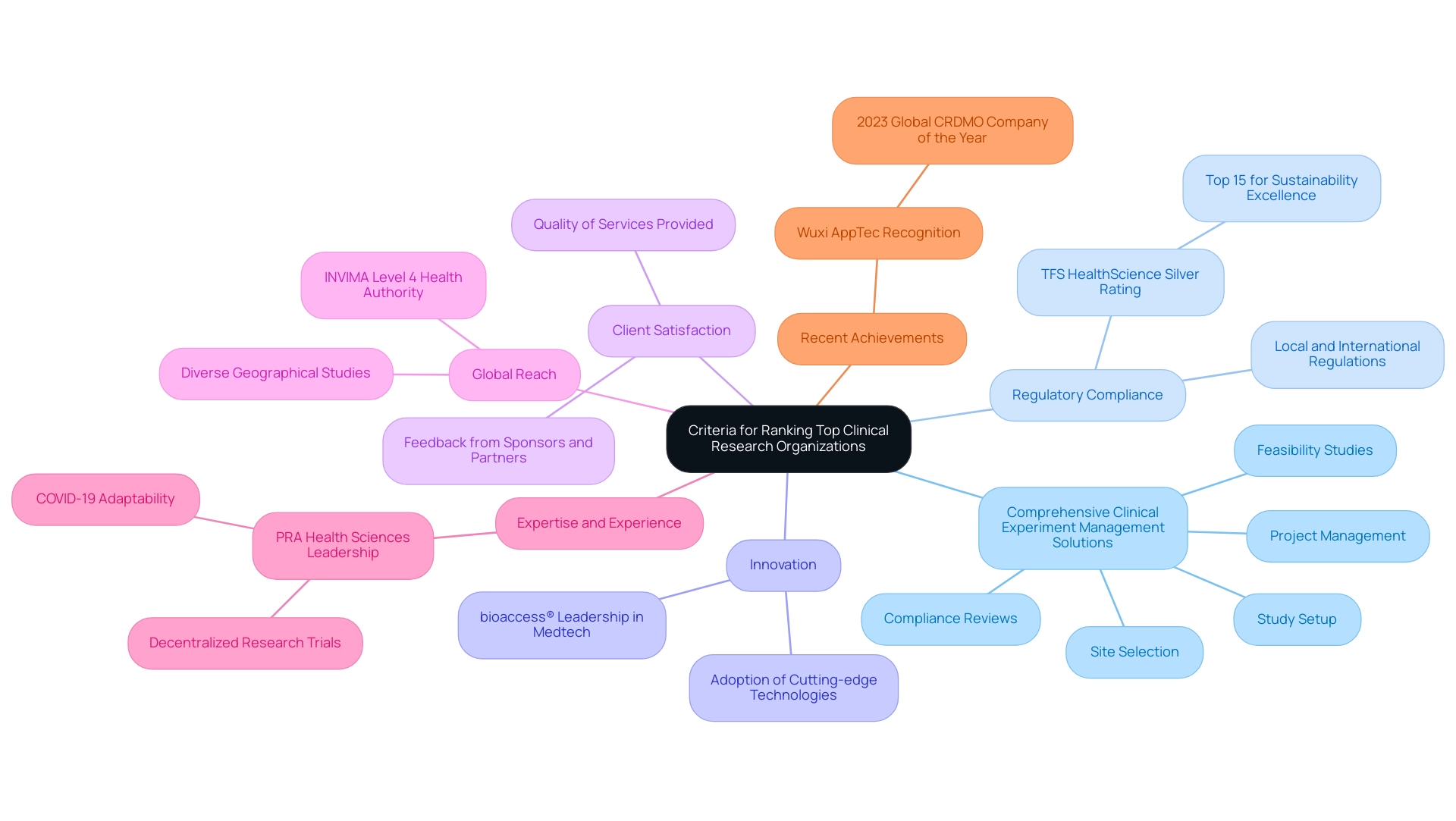
2. Criteria for Ranking Top Clinical Research Organizations
When evaluating the top 10 clinical research organizations in the world, several essential criteria emerge that highlight their capabilities and compliance with industry standards.
-
Comprehensive Clinical Experiment Management Solutions:
CROs should provide a complete range of offerings, including feasibility studies, site selection, compliance reviews, study setup, and project management. For instance, our service capabilities include the review and feedback on study documents to meet country requirements, setup and approval from ethics committees and health ministries, import permit facilitation, nationalization of investigational devices, and comprehensive reporting on study status, inventory, and both serious and non-serious adverse events.
-
Regulatory Compliance:
Adherence to both local and international regulations is crucial for ensuring the integrity and reliability of clinical studies. This commitment to compliance not only protects safety but also enhances the credibility of the research findings. As emphasized by TFS HealthScience,
For the fifth consecutive year, TFS received a Silver Rating from EcoVadis in 2024, placing it in the top 15% for sustainability excellence, a distinction that reinforces TFS’s commitment to corporate sustainability.
-
Innovation:
The ability to adopt and implement cutting-edge technologies and methodologies significantly enhances the efficiency of trials and the accuracy of data collection. Organizations like bioaccess® lead the way in Medtech clinical research in Latin America, focusing on innovation and regulatory excellence, which plays a pivotal role in optimizing clinical workflows and improving patient engagement.
-
Client Satisfaction:
Gathering feedback from sponsors and partners regarding the quality of services provided is indispensable. Elevated levels of client satisfaction can signify the success of tests and the overall effectiveness of the CRO's operations.
-
Global Reach:
A CRO’s ability to carry out studies across diverse geographical regions is vital. This global presence ensures access to a varied patient population and aids in navigating complex regulatory landscapes, thus enhancing compliance. The INVIMA, as a Level 4 health authority by PAHO/WHO, plays a crucial role in overseeing medical devices and ensuring that CROs meet necessary regulatory standards.
-
Expertise and Experience:
The depth of knowledge and practical experience in overseeing various types of studies across different therapeutic areas is a critical factor. Organizations such as PRA Health Sciences, now part of ICON plc, demonstrate this with their leadership in decentralized research trials, especially during the COVID-19 pandemic. Their innovative strategies in patient recruitment and retention highlight their adaptability and commitment to excellence.
-
Recent Achievements:
Wuxi AppTec, acknowledged as the 2023 Global CRDMO Company of the Year by Frost & Sullivan, demonstrates the competitive environment and recent accomplishments within the CRO sector, further highlighting the significance of remaining updated with industry advancements.
By taking these criteria into account, stakeholders can make informed choices when selecting from the top 10 clinical research organizations in the world to meet their research needs.
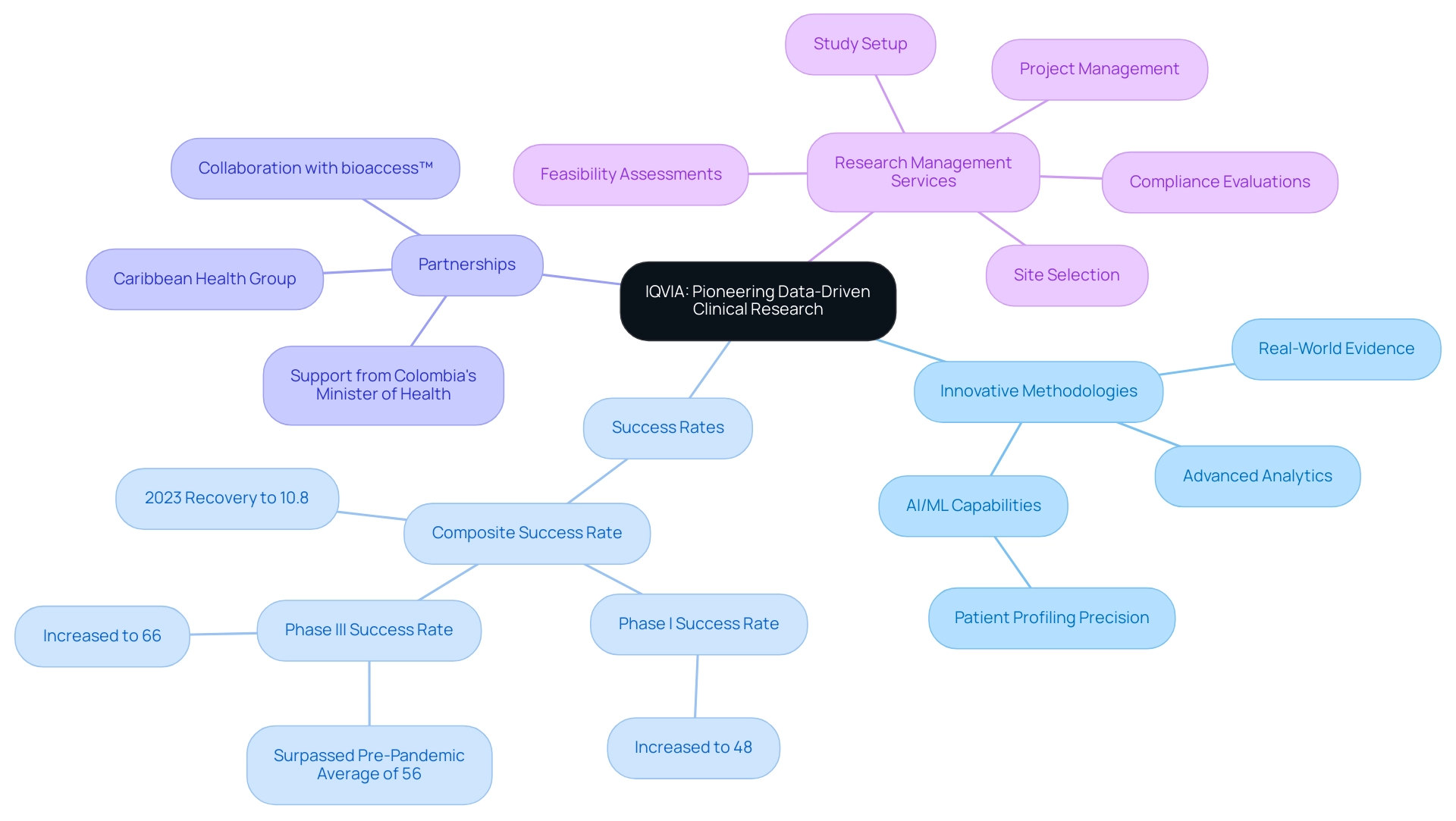
3. IQVIA: Pioneering Data-Driven Clinical Research
IQVIA sets itself apart as a leader in the research landscape, recognized for its innovative data-driven methodologies. Utilizing extensive healthcare data, IQVIA provides essential insights that significantly improve study design and execution. Their dedication to employing advanced technologies, including real-world evidence and sophisticated analytics, enables sponsors to make informed decisions throughout the development journey.
This strategic focus on data not only enhances efficiency but also improves patient recruitment and retention. Notably, the composite success rate for medical trials saw a remarkable recovery, reaching 10.8% in 2023, recovering from a 10-year low in 2022, partly attributed to IQVIA's efforts. Phase I success rates climbed to 48%, while Phase III rates surged to 66%, surpassing the pre-pandemic average of 56%.
This improvement underscores the effectiveness of IQVIA's methodologies. Furthermore, IQVIA has published over 200 AI scientific publications, showcasing their commitment to advancing AI in medical research. Their recent deployment of AI/ML capabilities to identify patient populations with unmet needs for a new respiratory disease treatment achieved an impressive 86% precision in patient profiling.
In partnership with bioaccess™ and Caribbean Health Group, IQVIA is also aiding in transforming Latin America into a premier location for research studies. Backed by Colombia's Minister of Health, these initiatives seek to improve research procedures in Barranquilla, attaining over a 50% decrease in recruitment duration and an impressive 95% retention rate. Furthermore, IQVIA's extensive research study management services encompass:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study setup
- Project management
ensuring thorough oversight and adherence to regulatory standards.
As Christina Mack, Chief Scientific Officer at Real World Solutions, articulates, 'At IQVIA, we are making this potential a reality, and are committed to going beyond the hype to ensure we’re taking a responsible approach, backed by scientific rigor, to get results that change people’s lives for the better.' These advancements highlight the significant influence of data-driven approaches in medical studies, paving the way for more successful outcomes.
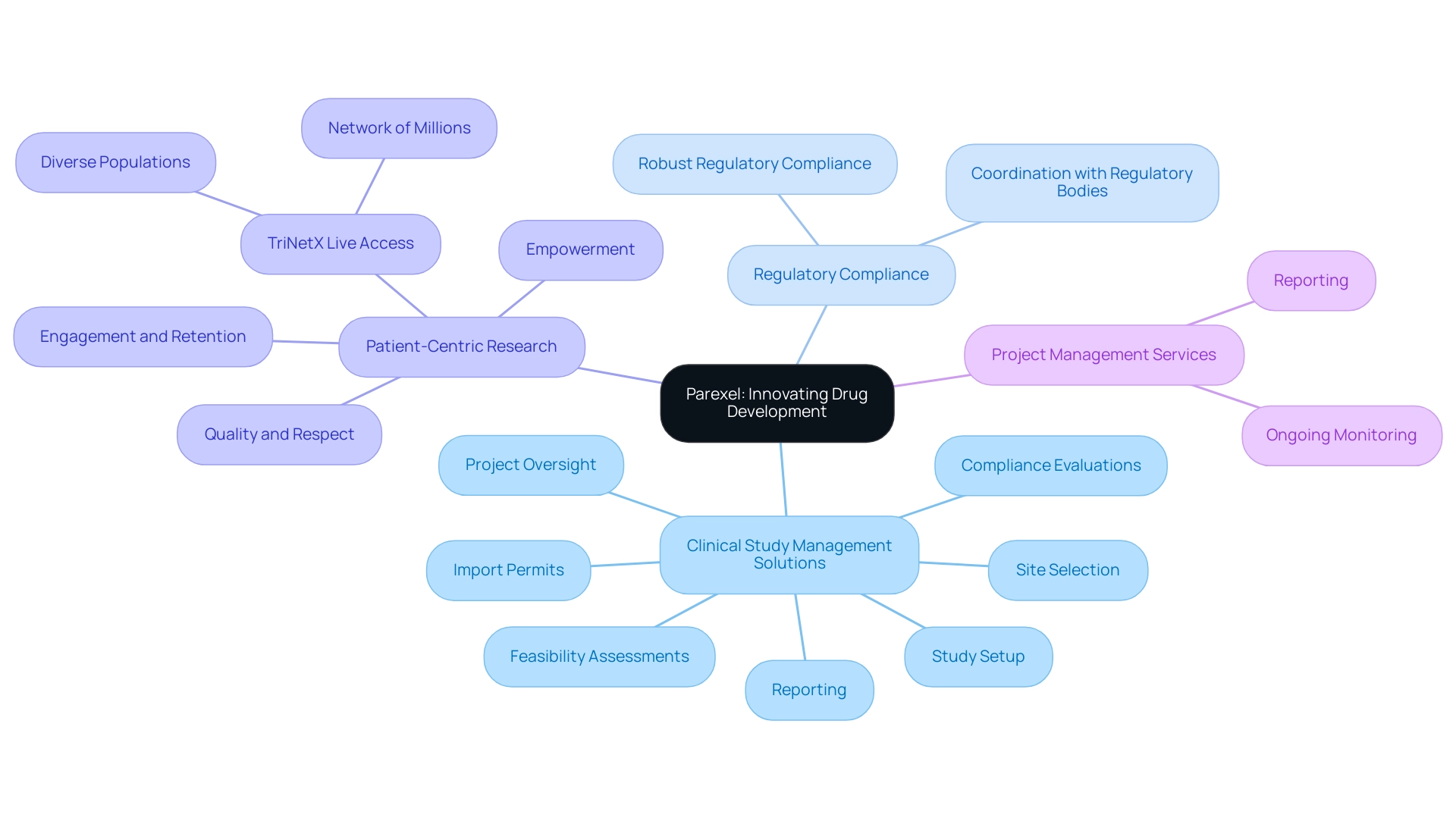
4. Parexel: Innovating Drug Development Processes
Parexel distinguishes itself in the research landscape with its innovative drug development strategies that significantly enhance the efficiency of research processes in Latin America. The company provides extensive clinical study management solutions, encompassing:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study setup
- Import permits
- Project oversight
- Reporting
Parexel's study setup process includes meticulous planning and coordination with local regulatory bodies to ensure compliance with country-specific requirements.
Their project management services encompass ongoing monitoring and reporting, which are essential for maintaining trial integrity and safety. With a global team of over 3,000 safety professionals, Parexel leverages nearly 40 years of expertise in pharmacovigilance to manage drug safety information effectively, ensuring robust regulatory compliance across diverse Latin American markets. Their emphasis on regulatory consulting and market access strategies facilitates the development of new therapies while addressing the unique challenges and opportunities present in the region.
A testament to their patient-first approach, Parexel focuses on quality, respect, and empowerment throughout their operations. Through TriNetX Live, they access a network of millions of individuals across more than 30 countries, allowing them to engage diverse populations crucial for effective research in Latin America. As Parexel states, 'Through TriNetX Live, we’re able to access a network of millions of patients in more than 30 countries, so you can access the diverse populations you need.'
This commitment to patient-centric research enhances participant engagement and retention, making Parexel a preferred partner for pharmaceutical companies eager to bring innovative drugs to market and driving global health improvement through international collaboration and innovation in medtech.
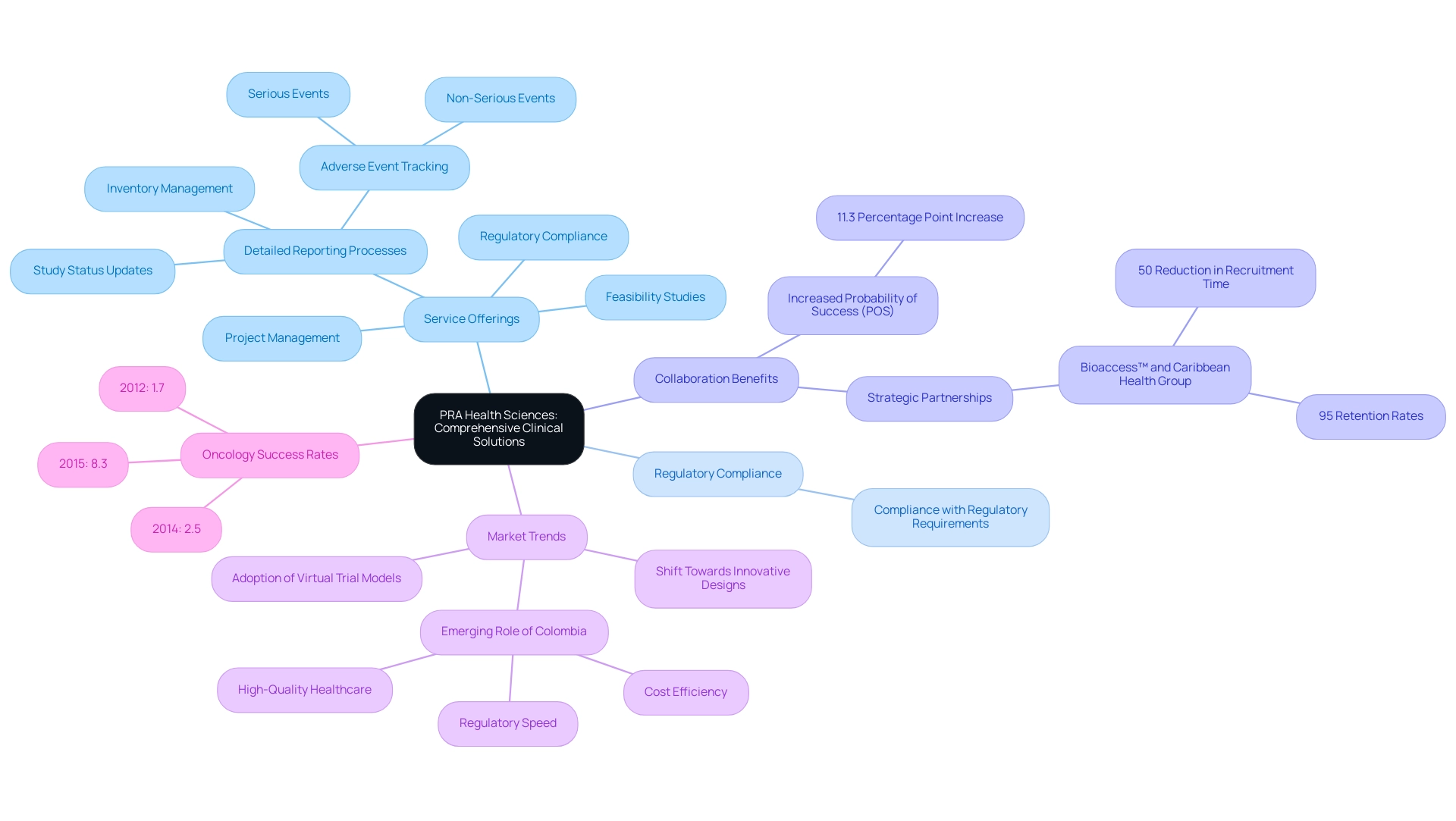
5. PRA Health Sciences: Comprehensive Clinical Solutions
PRA Health Sciences distinguishes itself in the clinical research field with its comprehensive clinical solutions that cover every phase of the process, from early-phase studies to post-marketing surveillance. Their diverse suite of services is meticulously tailored to meet the varied needs of clients, ensuring that operational excellence is consistently achieved. This encompasses:
- Feasibility studies
- Regulatory compliance
- Project management
- Detailed reporting processes
These elements are essential for progressing medical device evaluations efficiently.
Reporting encompasses:
- Study status updates
- Inventory management
- Tracking both serious and non-serious adverse events
This ensures compliance with regulatory requirements. A notable focus on patient involvement enhances study efficiency, ultimately driving high-quality results. Recent trends in the research studies market spotlight the shift towards innovative design formats and virtual models, areas where PRA excels.
Furthermore, Colombia's emerging role as a competitive destination for first-in-human trials is bolstered by:
- Cost efficiency
- Regulatory speed
- High-quality healthcare
This is supported by initiatives from Colombia's Minister of Health. Collaboration between bioaccess™ and Caribbean Health Group positions Barranquilla as a prominent research center in Latin America, achieving over 50% reduction in recruitment time and 95% retention rates, showcasing the benefits of strategic partnerships. As highlighted by Danzon, 'This result extends the findings by Danzon,' emphasizing the importance of collaboration in achieving operational excellence.
Furthermore, the oncology success rates have experienced a notable enhancement, from 1.7% in 2012 to 8.3% in 2015, highlighting the essential role of effective treatment solutions. A study on collaboration in drug development reveals that partnering with non-industry players can lead to an impressive 11.3 percentage point increase in the probability of success (POS), illustrating the benefits of collaboration in the pharmaceutical industry. PRA's worldwide presence as one of the top 10 clinical research organizations in the world enables studies in various areas, providing access to a diverse array of patient groups and strengthening their standing as a frontrunner in operational excellence within research.
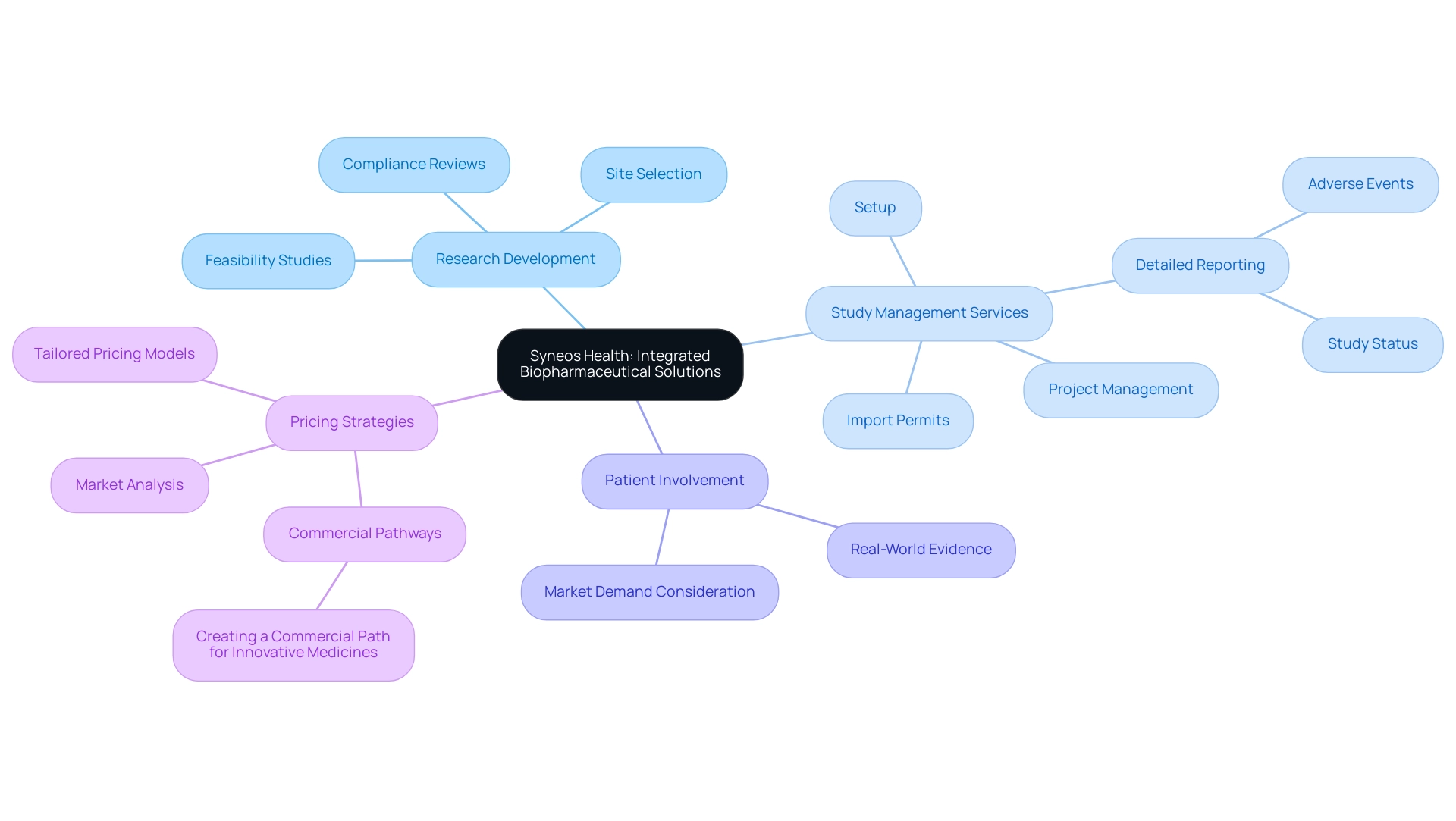
6. Syneos Health: Integrated Biopharmaceutical Solutions
Syneos Health sets itself apart through its inventive integrated biopharmaceutical solutions, effortlessly combining research development with extensive study management services. This encompasses:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Detailed reporting on study status and adverse events
Such a model not only promotes teamwork among groups but also guarantees that studies are crafted with market demands considered from the very start.
By prioritizing patient involvement and utilizing real-world evidence, Syneos Health improves the design and execution of studies, significantly increasing the chances of successful product launches. Furthermore, their adeptness in navigating complex regulatory environments empowers clients to meet their development objectives effectively. This strategic method not only enhances research processes but also aligns with the growing demand for integrated solutions in biopharmaceutical development, driving global health improvement through international collaboration and innovation in Medtech.
Additionally, as highlighted by Leslie Isenegger, Principal Strategist in Risk & Reputation Management, innovative pricing strategies are critical for new therapies, establishing commercial pathways essential for ensuring these therapies reach the market successfully. These strategies are implemented through thorough market analysis and tailored pricing models that enhance accessibility and affordability. A relevant case study titled 'Creating a Commercial Path for Innovative Medicines' illustrates Syneos Health's commitment to developing these pathways, showcasing their expertise in pricing strategies that enhance healthcare improvement and contribute to economic growth.
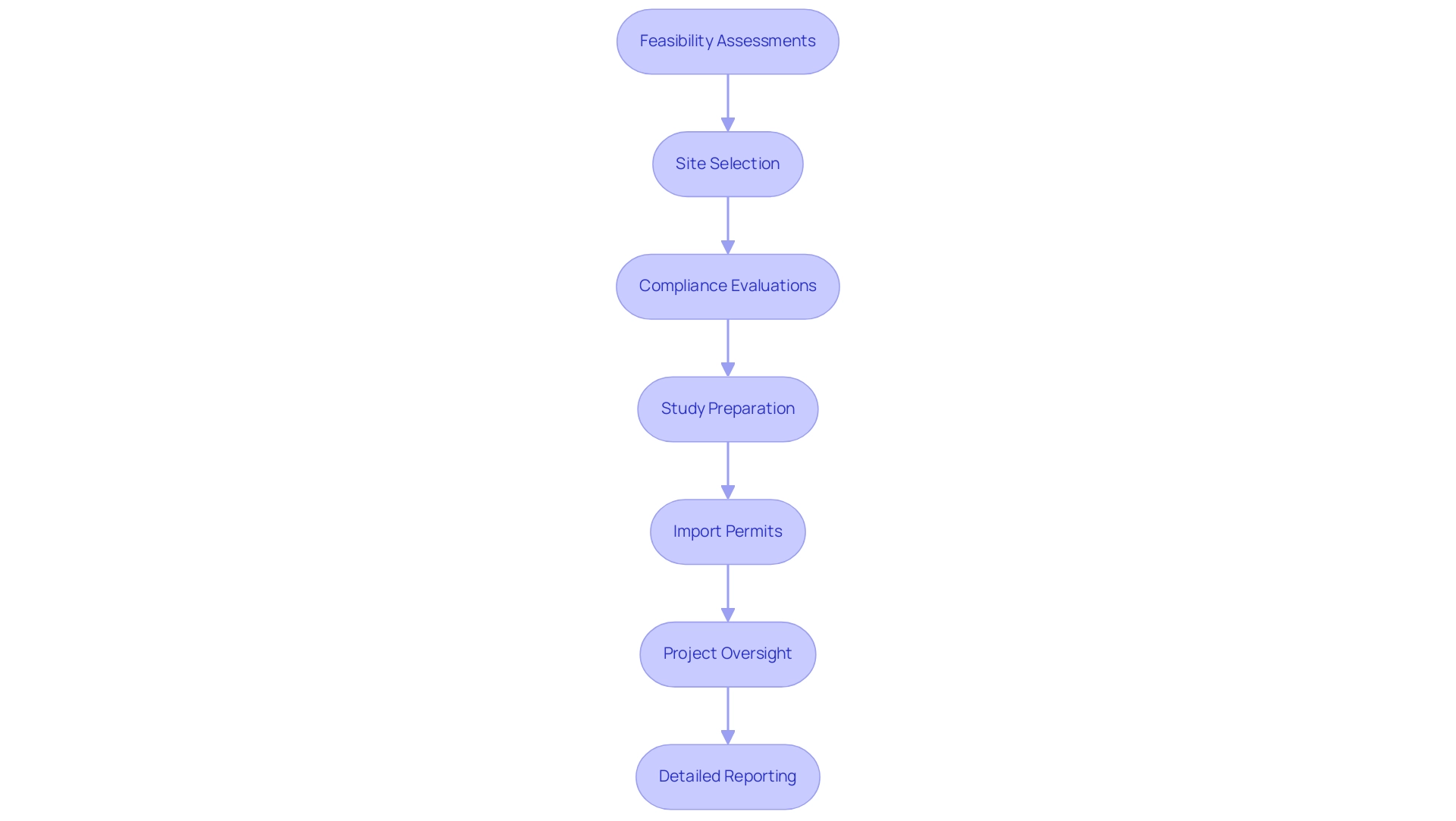
7. Charles River Laboratories: Advancing Drug Development
Charles River Laboratories stands out as a prominent force in advancing drug development, particularly during the critical preclinical phase. Their extensive study management offerings encompass:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study preparation
- Import permits
- Project oversight
- Detailed reporting on research status, inventory, and both serious and non-serious adverse events
This comprehensive knowledge in toxicology, pharmacology, and safety evaluation not only influences the structure of studies but also promotes global health advancement through international cooperation and innovation in the medtech sector.
Furthermore, their 2024 Financial Guidance Update, which reflects a narrowed revenue and non-GAAP earnings per share estimate, indicates a more optimistic outlook amidst recent challenges, such as a $215 million non-cash goodwill impairment affecting their GAAP operating margin. As of September 28, 2024, the company holds $899.3 million on its stock buyback authorization, demonstrating strong financial stability that supports their research management services. S&P Global Ratings has noted that, 'We could raise our ratings over the next 12-18 months if the company's operating performance experiences minimal volatility during this current period of industry stress.'
This resilience emphasizes their commitment to quality and innovation, solidifying their position as a trusted partner within the pharmaceutical industry, ultimately contributing to job creation, economic growth, and healthcare improvements in local economies.
8. ICON plc: Delivering Global Clinical Development
ICON plc distinguishes itself in the field of global development solutions, efficiently overseeing studies across various countries and regions since its inception in Latin and Central America in 1998. With a workforce of over 41,100 professionals spanning more than 55 countries, ICON utilizes a vast network of sites and investigators to enable efficient participant recruitment and data gathering. Their extensive research management offerings encompass:
- Feasibility studies
- Careful site selection
- Compliance assessments
- Study setup
- Import permits
- Nationalization of investigational devices
- Project management
This ensures adherence to local regulations while enhancing operational efficiency.
This robust infrastructure not only supports economic growth and job creation within local economies but also fosters healthcare improvement and international collaboration in Medtech. Their dedication to innovation is demonstrated by the recent launch of the ICON Digital Platform, which incorporates individuals, locations, and sponsor offerings, enabling customizable study designs that accommodate both conventional and entirely decentralized research. This platform utilizes functional cookies to enhance functionality and personalization, ensuring a smooth user experience.
As Steve Cutler, CEO of ICON, expresses, 'This latest version of the Digital Platform facilitates the effective capture and delivery of quality data from a variety of decentralized research services.' Its simplicity facilitates increased participant centricity, lowering the obstacles to research involvement and improving the equity, diversity, and inclusion of participant groups. The outcome of this platform not only improves patient participation but also significantly enhances equity, diversity, and inclusion among patient populations, thereby addressing critical gaps in research. By utilizing digital technologies and real-world evidence, ICON not only enhances study efficiency but also greatly improves data quality, establishing themselves as a leader in the research landscape.
Furthermore, their tailored services span the entire lifecycle of product development and commercialization, ensuring comprehensive support from initial concept to market launch, thereby facilitating effective market access strategies in the Latin American Medtech landscape. Moreover, bioaccess® plays a crucial role in these initiatives by supporting medical device evaluations in the region, further strengthening ICON's abilities in maneuvering through the intricate landscape of Latin American healthcare.
![]()
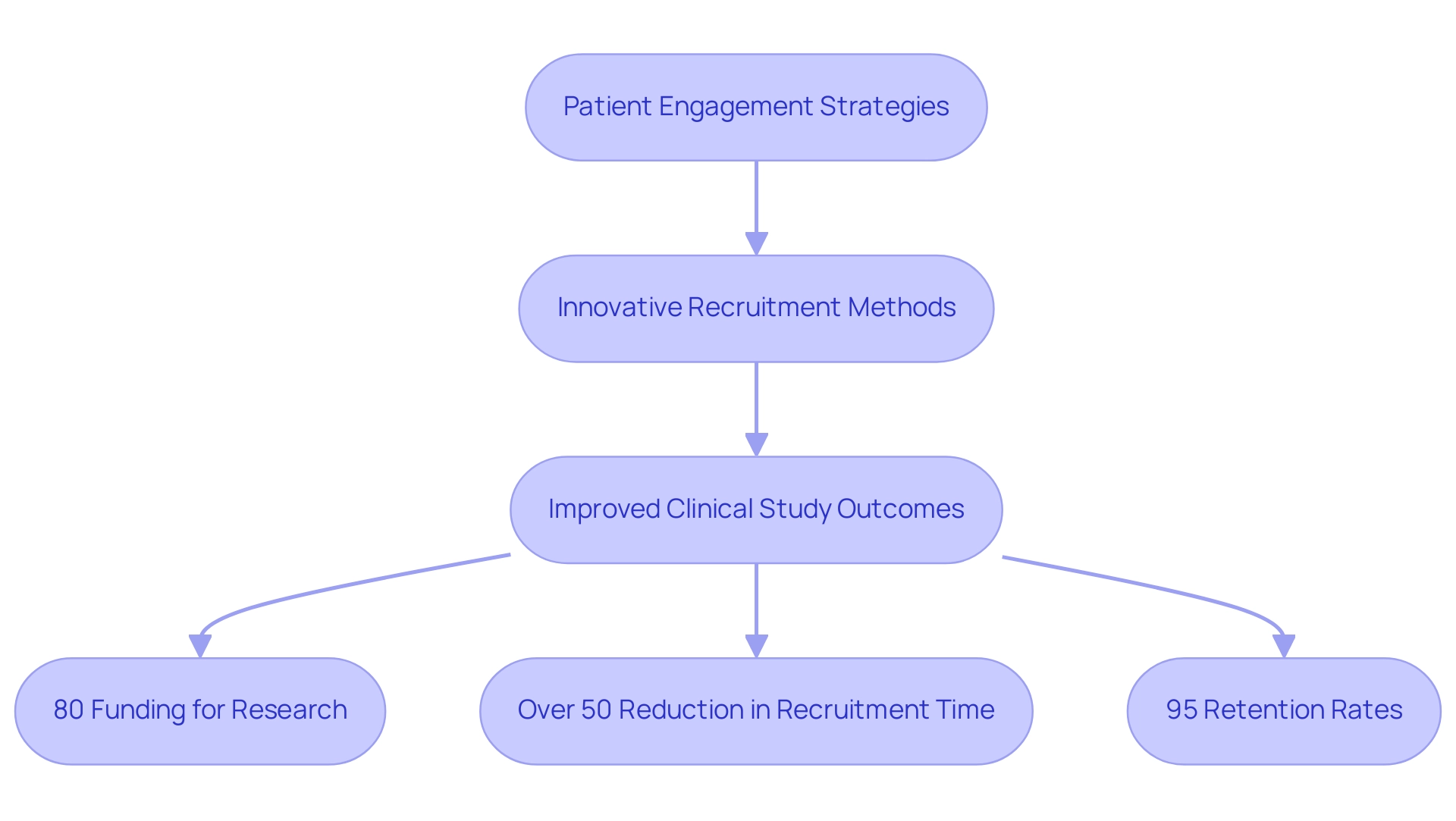
9. Labcorp Drug Development: Patient-Centric Research
Labcorp Drug Development demonstrates a robust dedication to research focused on individuals, recognizing that involvement from those receiving care greatly impacts the effectiveness of clinical studies. Recent studies indicate that effective engagement can lead to better trial outcomes and increased satisfaction among participants. Approximately 80% of the PCOR Trust Fund funding is allocated for research, underscoring the importance of prioritizing participant involvement.
Labcorp employs innovative recruitment strategies, leveraging digital tools and community outreach initiatives to enhance involvement. As Vg Vinod Vydiswaran, Assistant Professor in the Department of Learning Health Sciences, emphasizes, 'Developing and applying methodologies for extracting relevant information from health-related text corpora is crucial for understanding the needs of individuals.' By concentrating on the particular requirements and preferences of individuals, Labcorp guarantees that research studies are structured with the experiences of participants as a priority.
This method not only promotes a more inclusive research environment but also speeds up drug development timelines, ultimately benefiting both individuals and the broader healthcare landscape. Moreover, the current conversations regarding revising data privacy laws emphasize how legislative modifications can influence patient involvement and data exchange in research studies. In Colombia, the partnership between bioaccess™ and Caribbean Health Group seeks to turn the region into a premier location for research studies in Latin America, backed by initiatives from the Minister of Health.
Through comprehensive services including feasibility studies, site selection, compliance reviews, setup for testing, import permits, and project management, this partnership aims to improve research efficacy. Notably, it has achieved over a 50% reduction in recruitment time and maintains impressive 95% retention rates, demonstrating its effectiveness. As the landscape of medical research continues to evolve, Labcorp remains at the forefront, championing strategies that prioritize patient engagement as a key driver of success while also contributing to the advancement of medtech in Latin America.
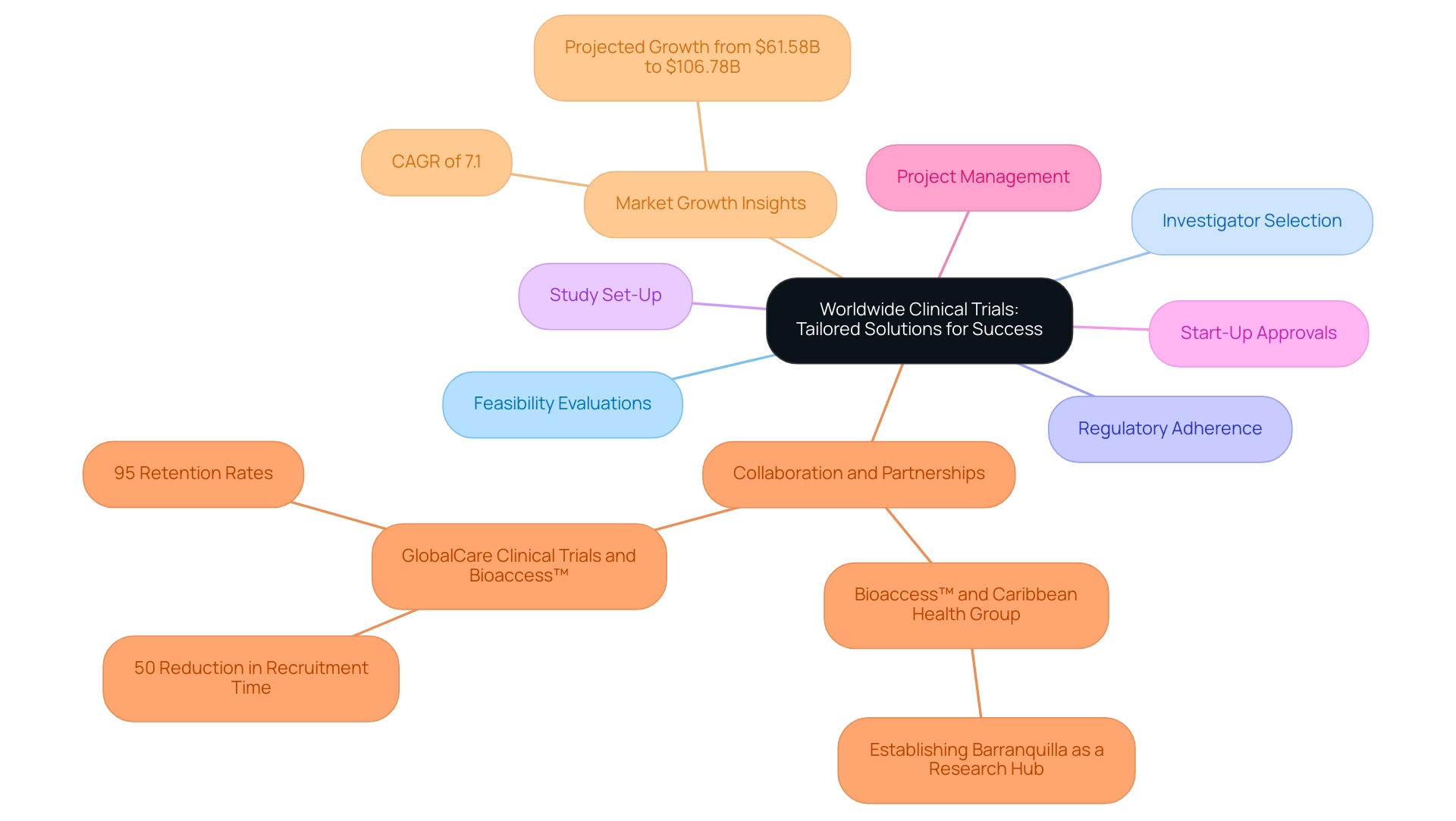
10. Worldwide Clinical Trials: Tailored Solutions for Success
Worldwide Clinical Trials has built a strong reputation for providing customized solutions that meet the unique requirements of each client, especially in managing the complexities of advancing medical device studies. Their comprehensive services encompass:
- Feasibility evaluations
- Investigator selection
- Regulatory adherence
- Study set-up
- Start-up approvals
- Project management
These services ensure that research studies are meticulously designed and executed to align with unique objectives and challenges. Additionally, they provide review and feedback on study documents to comply with country requirements.
This tailored approach is further enhanced by their emphasis on collaboration and open communication, fostering robust partnerships with sponsors and significantly improving the overall experience. The partnership between bioaccess™ and Caribbean Health Group to establish Barranquilla as a prominent location for research in Latin America, backed by Colombia's Minister of Health, illustrates the worldwide movement towards strategic alliances. Furthermore, GlobalCare Clinical Trials' partnership with bioaccess™ has achieved notable efficiencies, including a 50% reduction in recruitment time and 95% retention rates, showcasing how tailored approaches can address challenges faced by medical device startups.
As the global trials market is projected to grow from $61.58 billion in 2024 to $106.78 billion by 2032, at a CAGR of 7.1%, organizations like Worldwide Trials are well-positioned to leverage this growth by continually enhancing their service offerings and capabilities. Their dedication to quality and efficiency solidifies their role as a valuable partner in the clinical research landscape, driving successful outcomes for clients.
Conclusion
The role of Clinical Research Organizations (CROs) in the landscape of clinical trials is increasingly vital as they address the complexities of drug development and medical device innovation. Throughout this article, key functions of CROs have been highlighted, including:
- Comprehensive management services
- Regulatory compliance
- Innovative approaches that enhance trial efficiency
The significant partnerships and achievements of industry leaders such as IQVIA, Parexel, and Labcorp exemplify how these organizations are not only improving recruitment and retention rates but also transforming the clinical research ecosystem through patient-centric strategies and advanced technologies.
Selecting the right CRO is crucial for stakeholders aiming to navigate the intricate challenges of clinical research. Criteria such as:
- Comprehensive service offerings
- Regulatory adherence
- Commitment to innovation
should guide this selection process. The article has showcased various CROs that are setting benchmarks in the industry, demonstrating the importance of expertise and experience in achieving successful trial outcomes.
In summary, as the demand for effective therapies grows, CROs will continue to be indispensable partners in clinical research. Their ability to streamline processes, ensure compliance, and enhance patient engagement positions them as key players in advancing healthcare innovations. Embracing these partnerships and understanding the pivotal role of CROs will empower stakeholders to drive successful clinical trials and ultimately improve patient outcomes in an ever-evolving medical landscape.
Frequently Asked Questions
What are the main services provided by the top clinical research organizations (CROs)?
The top clinical research organizations provide a range of services including feasibility studies, selection of research locations and lead investigators, regulatory compliance, study setup, import permits for investigational devices, and rigorous project management and reporting.
How do CROs contribute to drug development?
CROs facilitate the development of new therapies by ensuring adherence to regulatory standards, providing strategic guidance throughout the research process, and managing the complexities of clinical studies.
What recent collaboration highlights the effectiveness of CROs?
The collaboration between GlobalCare Clinical Trials and bioaccess™ demonstrated a reduction of over 50% in subject recruitment duration and a retention rate exceeding 95% in Colombia, showcasing the effectiveness of CROs in enhancing research processes.
What challenges do medical device startups face in clinical research?
Medical device startups often encounter challenges such as regulatory hurdles, competition, recruitment issues, and financial constraints.
How do CROs help address challenges in medical research?
CROs play an essential role in navigating regulatory complexities, managing ethical concerns, and providing support to overcome challenges faced by startups in clinical research.
What role does AI play in modern clinical studies?
AI is increasingly incorporated into medical studies to improve disease identification and enhance data analysis, as exemplified by the partnership between IDx Technologies and bioaccess™.
Why is transparency in clinical study costs important?
Transparency in clinical study costs is crucial for effective financial management and ethical research practices, as indicated by a cost analysis revealing an average expenditure of approximately 72,000 USD per study in Switzerland.
What criteria should be considered when evaluating CROs?
Key criteria include comprehensive clinical experiment management solutions, regulatory compliance, innovation, client satisfaction, global reach, expertise and experience, and recent achievements.
What distinguishes IQVIA in the research landscape?
IQVIA is recognized for its innovative data-driven methodologies, extensive healthcare data utilization, and a strong focus on advanced technologies to improve study design and execution.
What success rates have been observed in medical trials due to IQVIA's methodologies?
The composite success rate for medical trials reached 10.8% in 2023, with Phase I success rates at 48% and Phase III rates at 66%, demonstrating a significant recovery and improvement in trial outcomes.
What specific management services does IQVIA offer?
IQVIA offers services such as feasibility assessments, site selection, compliance evaluations, study setup, and project management to ensure thorough oversight and regulatory adherence.

