Introduction
In the complex landscape of medical device regulation, understanding the nuances of 510(k) submissions is paramount for manufacturers seeking to bring their innovations to market. This regulatory pathway, which serves as a premarket notification to the FDA, requires a comprehensive demonstration of a device's safety and effectiveness in comparison to a predicate device.
With the stakes high, manufacturers must navigate a myriad of requirements, from substantial equivalence assessments to meticulous documentation processes. The involvement of regulatory experts, such as Ana Criado and Katherine Ruiz, is crucial in guiding companies through this intricate terrain, ensuring compliance and facilitating efficient market entry.
As the medical device industry continues to evolve, grasping the intricacies of 510(k) submissions will be essential for manufacturers aiming to succeed in a competitive environment.
Understanding 510(k) Submissions: A Regulatory Overview
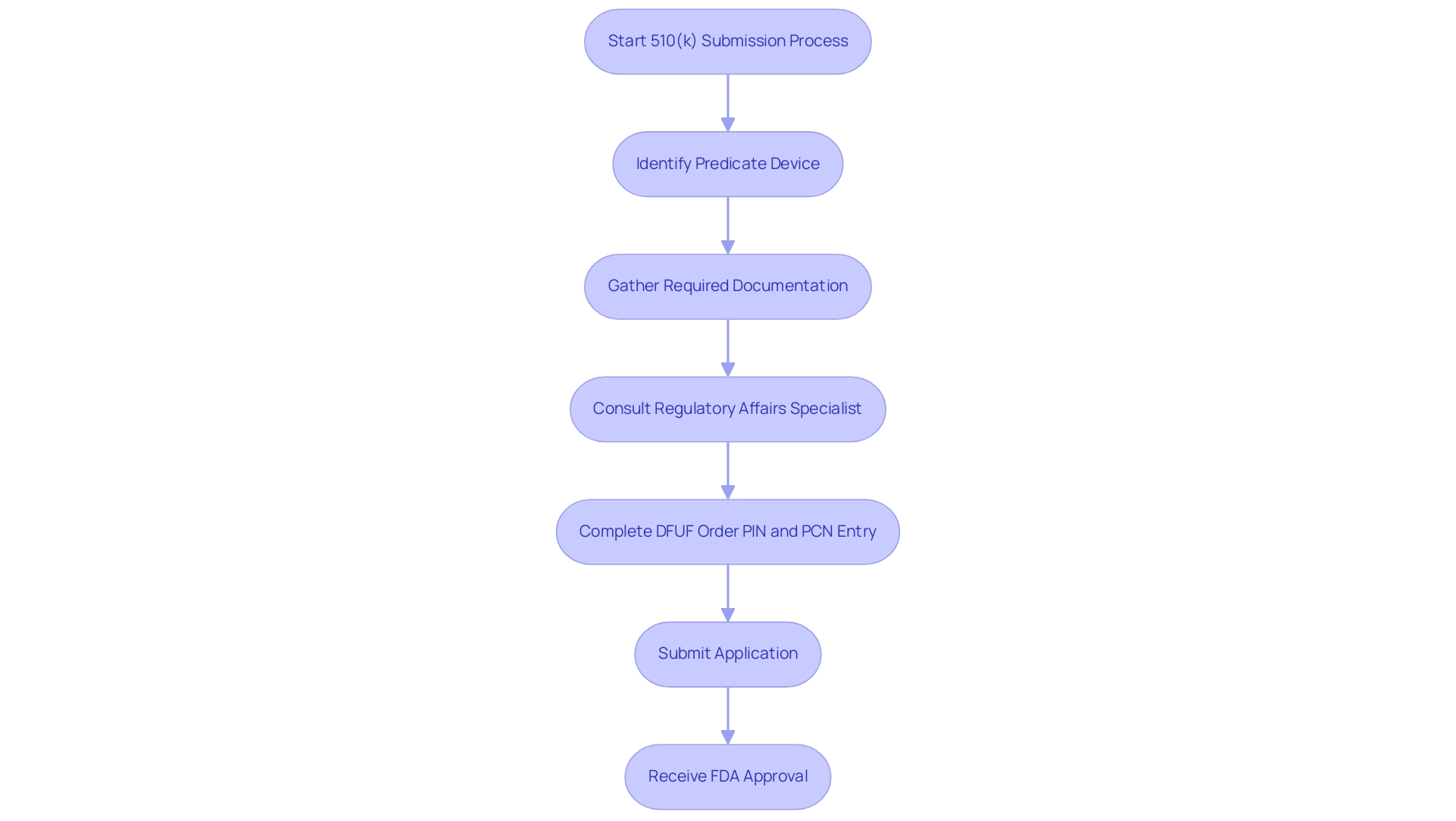
The 510k device application functions as a vital premarket notification to the FDA, enabling manufacturers to showcase the safety and effectiveness of their medical products. This entry is mandated for devices that are substantially equivalent to a previously marketed device, known as a predicate device. Navigating the intricate regulations surrounding the 510k device submissions is essential for manufacturers, as it delineates the specific documentation and data needed for approval.
Specialists in Regulatory Affairs, like Ana Criado and Katherine Ruiz, play a crucial role in assisting manufacturers through this procedure, utilizing their extensive knowledge in biomedical engineering and regulatory compliance. The procedure requires a thorough assessment of the system's design, intended use, and performance traits, ensuring compliance with the rigorous FDA standards for safety and effectiveness. A significant requirement in this procedure is the entry of the DFUF order PIN and PCN after completing registration and device listing, which is critical for ensuring that the registration is properly recorded and processed.
For instance, in the case study titled 'Entering DFUF Order PIN and PCN,' establishments that followed this step were able to finalize their registration effectively, highlighting its importance. Additionally, the fee for a 513(g) request for classification information stands at $6,528, representing one of the financial considerations manufacturers must account for. Furthermore, as emphasized in the recent updates, there will be no small business waiver for the annual establishment registration fee for FY 2024, meaning all establishments must pay the same fee, thereby highlighting the importance of understanding the financial aspects of the 510k device process.
According to recent statistics, the approval rates for submissions of the 510k device in 2024 indicate a trend of increasing efficiency, with many manufacturers successfully navigating the procedure within an average timeline of 90 days. By meticulously following these guidelines, manufacturers can ensure their products are appropriately vetted and ready for market entry.
The Importance of Substantial Equivalence in 510(k) Submissions
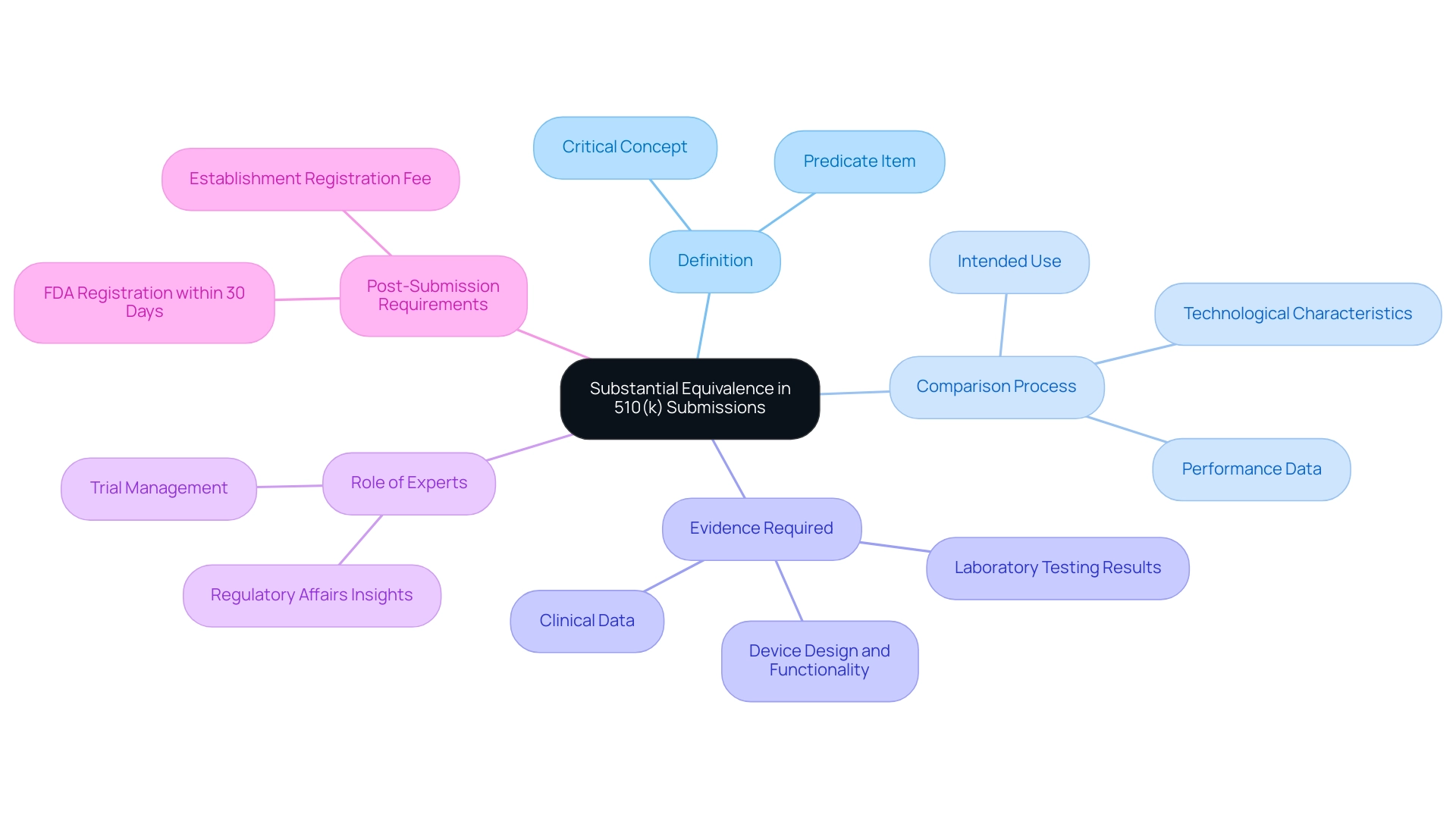
Substantial equivalence is a critical concept in the medical equipment approval process, defined as the determination that a new product is at least as safe and effective as a previously cleared predicate item. This assessment involves a detailed comparison of the new item's intended use, technological characteristics, and performance data against those of the predicate item. Significantly, products introduced after May 28, 1976, must demonstrate substantial equivalence to be exempt from pre-market approval, underscoring its importance in regulatory compliance.
Manufacturers are tasked with providing robust evidence to substantiate their claims of substantial equivalence. This evidence may comprise clinical data, laboratory testing results, and comprehensive descriptions of the device's design and functionality. As stated by the FDA, in cases where manufacturers determine under their design control procedures that no additional verification or validation testing is necessary to evaluate a change that otherwise requires the filing and clearance of a 510k device, manufacturers may submit these changes as a Special 510k device with a clear rationale supporting their conclusion that no performance data are necessary.
A thorough understanding of substantial equivalence is paramount, as it significantly affects the outcome of the 510k device submission process. The capability to convincingly illustrate this equivalence for a 510k device can accelerate regulatory assessment and ease market entry, making it a focal point for manufacturers navigating the complexities of medical product approval. Furthermore, experts like Ana Criado, Director of Regulatory Affairs and founder of Mahu Pharma, provide invaluable insights into the regulatory landscape, particularly in Colombia, where they navigate compliance reviews, feasibility studies, trial setup, project management, and reporting for clinical trials.
Ana's expertise ensures that all necessary steps are taken for successful trial execution, including obtaining import permits and managing study logistics. Additionally, after obtaining clearance, companies must register their products with the FDA within 30 days, which includes an establishment registration fee for new companies. Awareness of these post-submission requirements ensures compliance with FDA regulations and allows for legal market entry.
Types of 510(k) Submissions: Traditional, Abbreviated, and Special
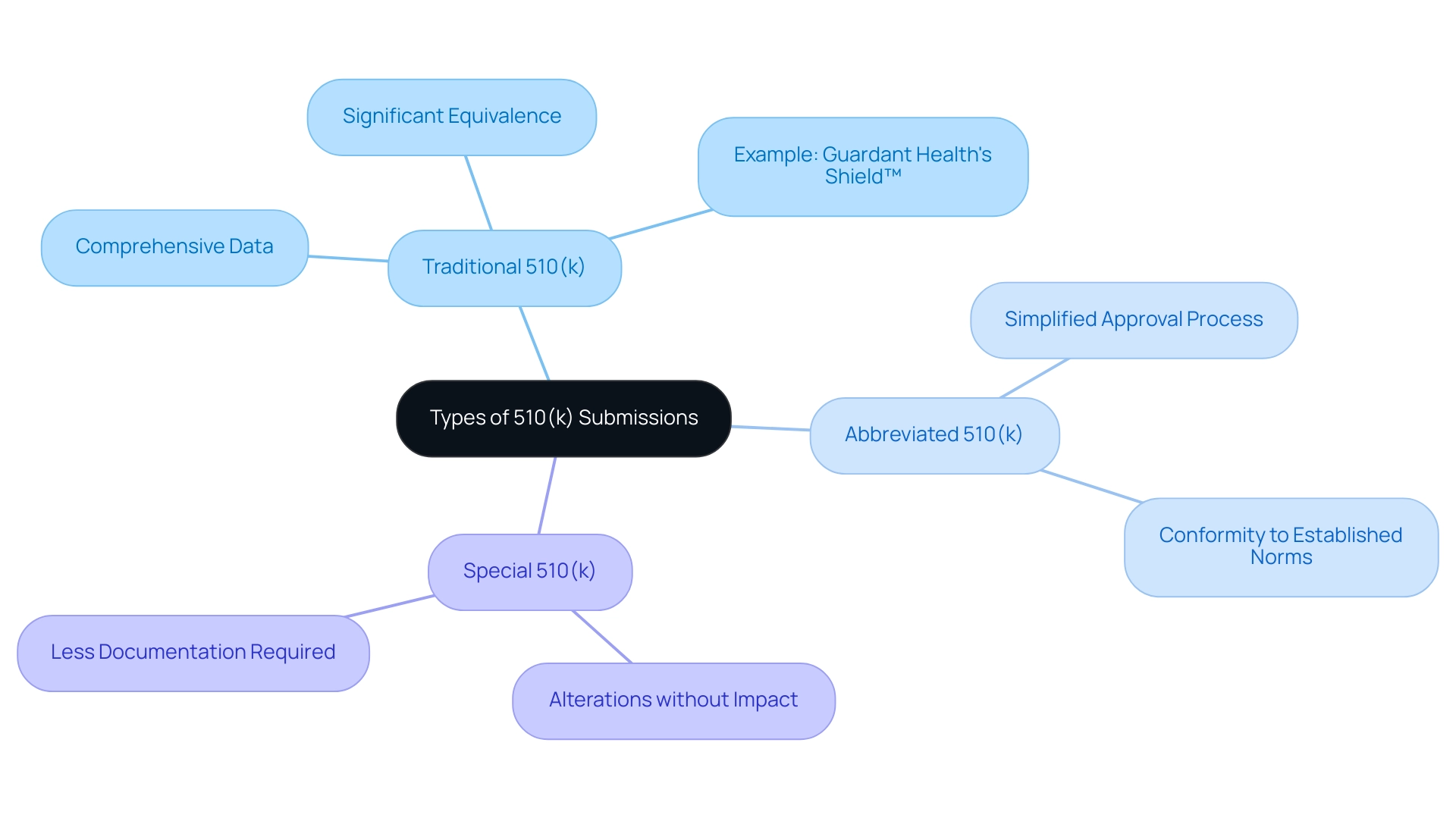
The process for filing a 510k device includes three main categories: Traditional, Abbreviated, and Special, each addressing different product features and filing requirements. The Traditional 510k device is the most common type of application, requiring manufacturers to furnish a comprehensive array of data and documentation. This comprehensive documentation is crucial for showcasing significant equivalence to a legally marketed product, ensuring that the new 510k device meets the required safety and effectiveness standards.
For instance, Guardant Health’s Shield™ blood test was approved by the FDA on July 31, 2024, for colorectal cancer screening in adults age 45 and older, exemplifying a successful Traditional 510(k) submission.
- The 510k device pathway is intended for products that fulfill specific criteria outlined by the FDA. By permitting manufacturers to refer to established guidance documents and acknowledged standards, the Abbreviated 510(k) simplifies the approval procedure, thereby decreasing the time and resources needed for authorization.
This option proves beneficial for equipment that can clearly demonstrate conformity to established norms, expediting their entry into the market. The Special 510(k) device application is designed for 510k devices that experience alterations not impacting their intended use or technological traits, providing a more efficient review method. It requires less documentation than the Traditional 510k device, enabling faster approval times while still ensuring compliance with regulatory standards.
As noted by Roseann White, Co-Chair of the AdvaMed Statistical Working Group, understanding these pathways is crucial for manufacturers aiming to navigate the complexities of the 510k device approval process effectively.
Insights from professionals like Ana Criado, the Director of Regulatory Affairs and a consultant with extensive experience in biomedical engineering and regulatory frameworks, underscore the importance of these distinctions. Ana, who heads Mahu Pharma and has held multiple regulatory positions, stresses that her expertise can greatly assist manufacturers in managing these application types, especially in coordinating their strategies with the recent guidance, 'Evidentiary Expectations for 510(k) Implant Devices.' This guidance acts as a primary resource for general recommendations on all implant products requiring a 510k device, providing essential context on current regulatory expectations.
Additionally, Katherine Ruiz, an expert in Regulatory Affairs for Medical Equipment and In Vitro Diagnostics in Colombia, highlights the collaborative efforts needed to address the evolving landscape of medical equipment regulation, further emphasizing the importance of understanding these pathways. Identifying these differences is crucial for producers looking to select the most appropriate category for their medical products, ultimately affecting the effectiveness of the approval procedure.
Navigating Challenges: Common Pitfalls in 510(k) Submissions
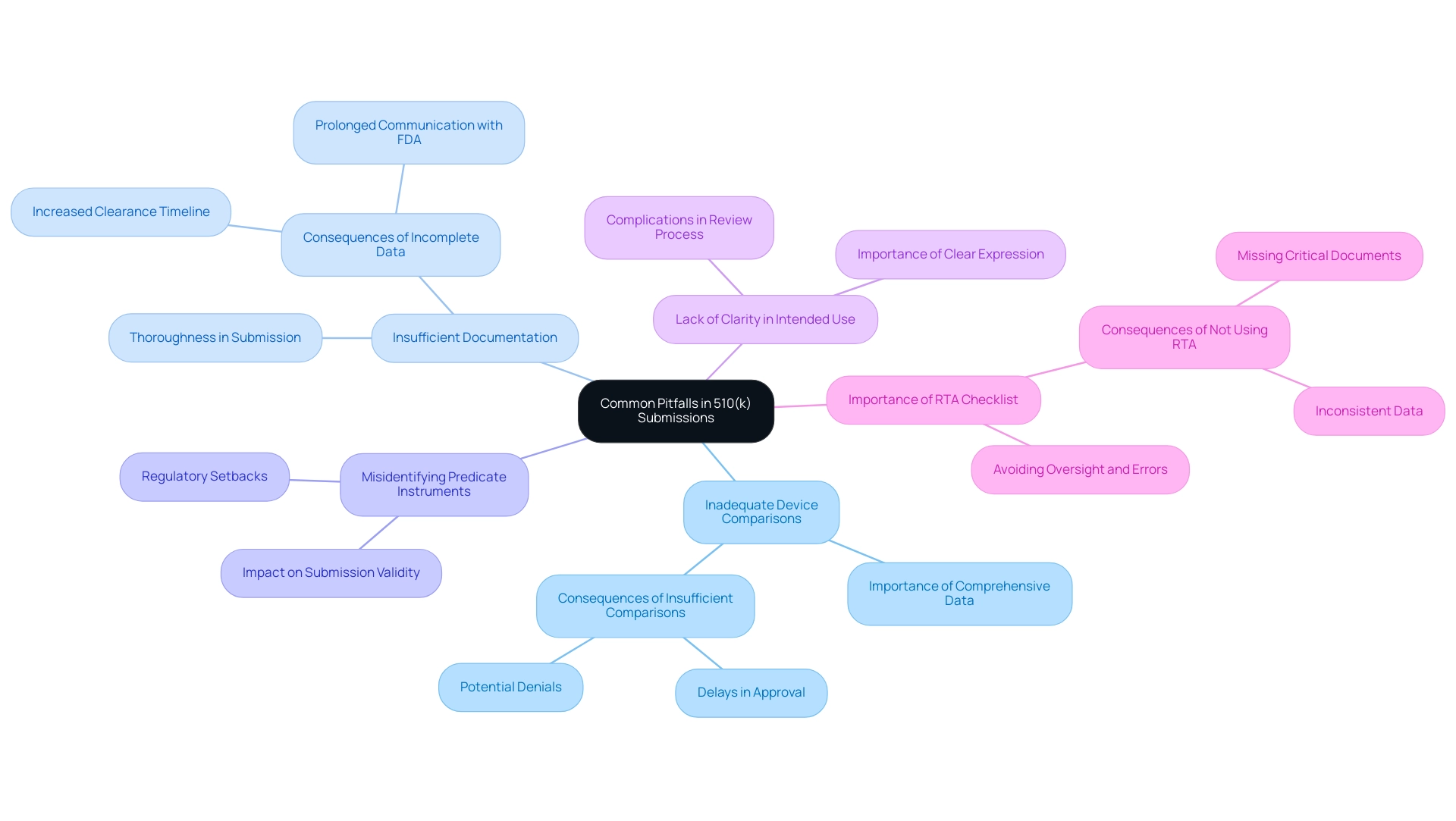
Manufacturers frequently encounter a series of challenges during the 510k device application process, which can complicate or even derail their efforts to gain regulatory clearance. Awareness of the following common pitfalls is crucial for improving submission quality and reducing potential complications:
- Inadequate Device Comparisons: Submitting insufficient data to demonstrate substantial equivalence to a predicate device can lead to significant delays or outright denials. It is essential to provide comprehensive comparisons that illustrate how the new product aligns with existing technologies. Once the FDA determines that the device is substantially equivalent, it issues a clearance letter allowing the manufacturer to market the device in the U.S.
- Insufficient Documentation: Omitting required documentation or presenting incomplete data can severely hinder the review process. A lack of thoroughness in documents may result in prolonged back-and-forth communication with the FDA, further delaying the clearance timeline. As Mike Drues from Vascular Sciences emphasizes, "Mistake #7 - Formatting Submission Incorrectly: Include page numbers and an eCopy. Also, the size of the file matters and needs to be named according to conventions."
- Misidentifying Predicate Instruments: Choosing the incorrect predicate instrument can compromise the validity of the submission. This misstep can lead to regulatory setbacks, as the FDA may question the appropriateness of the comparison, impacting the overall evaluation of the product's safety and effectiveness.
- Lack of Clarity in Intended Use: Ambiguities surrounding the intended application of a tool can complicate the review process. Clear expression of the product's purpose and use is vital for aiding the FDA's comprehension and assessment of the proposal.
Additionally, not using the RTA checklist during the filing of a 510k device can lead to oversight, errors, and omissions, resulting in missing critical documents or information. Possible outcomes encompass inconsistent data, failure to meet regulatory requirements, and inquiries regarding the safety and effectiveness of the product.
By proactively tackling these potential challenges, manufacturers can improve the quality of their entries, expedite the approval procedure, and reduce the risk of delays or refusals. This expertise is exemplified by regulatory leaders like Ana Criado, who, with her extensive background in biomedical engineering, health economics, and Regulatory Affairs, alongside her role as a professor and CEO of Mahu Pharma, emphasizes the importance of thorough preparation and understanding of the regulatory landscape in overcoming these challenges.
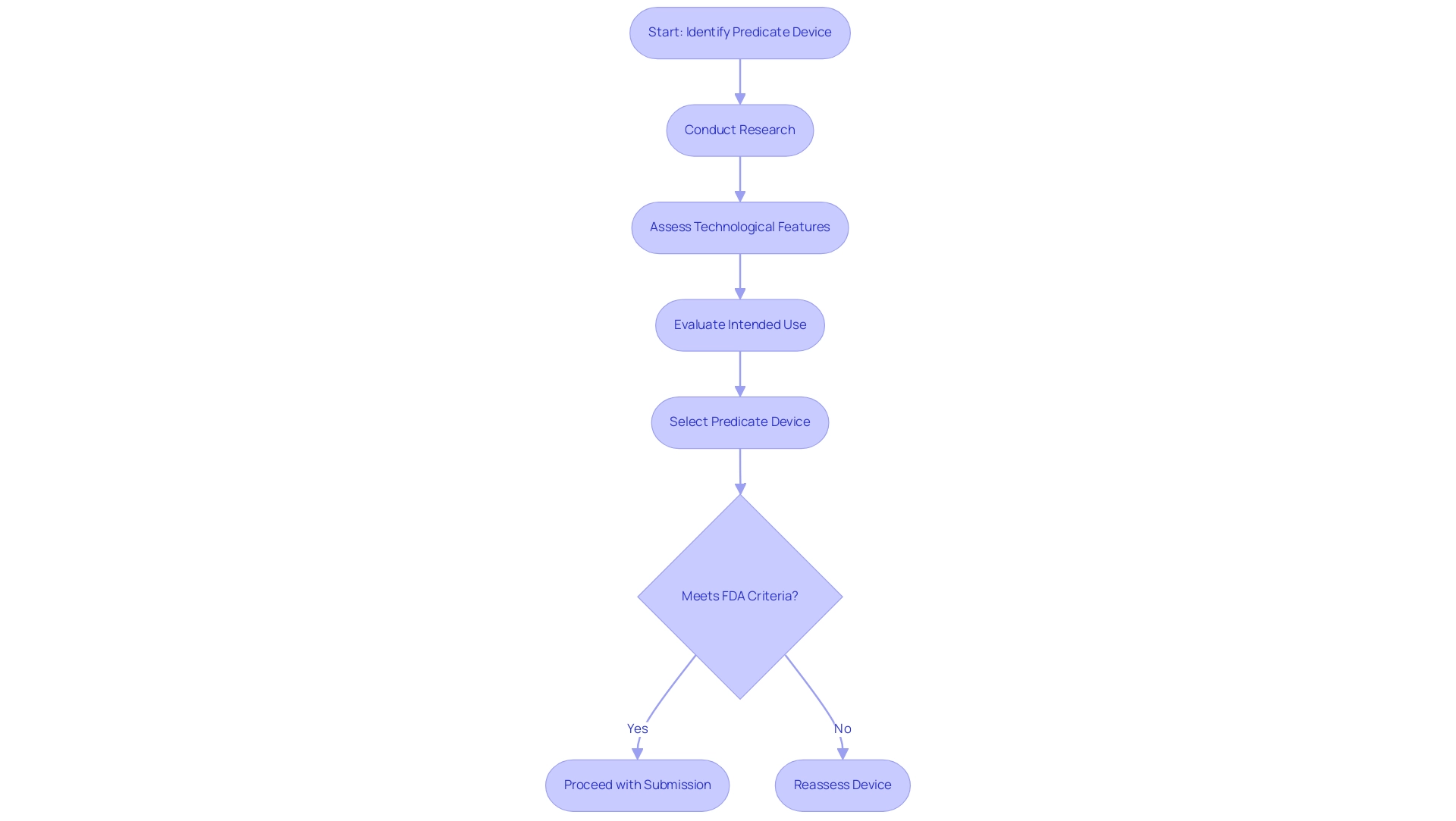
Identifying Predicate Devices: A Key Step in the 510(k) Process
Determining the appropriate predicate product is a crucial step in the 510k device submission procedure, with insights from specialists such as Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma. Ana's extensive experience at INVIMA, where she navigated complex regulatory frameworks, equips her with a deep understanding of the 510(k) process. Her academic background in biomedical engineering further enriches her insights, particularly in assessing the technological aspects of instruments.
A 510k device must be legally marketed and exhibit characteristics similar to those of the new apparatus, particularly regarding intended use and technological features. To ensure compliance with the FDA's criteria for substantial equivalence, manufacturers should undertake comprehensive research to identify a suitable predicate for their 510k device. The importance of selecting the appropriate predicate apparatus, like the 510k device, cannot be overstated; it affects the success rate of the proposal and shapes the overarching development strategy for the new apparatus.
Effective identification can streamline the review process, thereby facilitating a more efficient pathway to market. As noted by the Government Accountability Office (GAO), approximately 1% of the 5,063 Class II and III submissions reviewed by the FDA between 2005 and 2007 involved a new intended use, highlighting the critical nature of precise identification of instruments. Small manufacturers, typically defined as those with annual revenues of less than $30 million, face unique challenges in this area.
Obstacles such as the prohibition on third-party review organizations offering regulatory consulting can hinder effective predicate device selection. However, permitting these organizations to assist could boost participation in the review and yield improved outcomes. A case study on regulatory consulting for small manufacturers illustrates that tailored guidance can significantly improve their ability to navigate the complexities of the 510k device process.
The evolving landscape of predicate device selection necessitates ongoing attention to FDA guidelines and emerging best practices to optimize the chances of successful submissions, a sentiment echoed by regulatory experts like Katherine Ruiz, who specializes in medical devices and in vitro diagnostics in Colombia.
Conclusion
Navigating the complexities of 510(k) submissions is critical for medical device manufacturers aiming to achieve regulatory clearance and successfully enter the market. The process hinges on demonstrating substantial equivalence to a predicate device, requiring meticulous documentation and a clear understanding of the FDA's stringent safety and effectiveness standards. As highlighted, manufacturers face several challenges, from inadequate comparisons to misidentifying predicate devices, which can significantly impact the approval timeline and overall success of their submissions.
The importance of engaging regulatory experts, such as Ana Criado and Katherine Ruiz, cannot be overstated. Their insights and guidance are invaluable in overcoming common pitfalls and ensuring compliance with evolving regulations. By leveraging their expertise, manufacturers can enhance the quality of their submissions, streamline the approval process, and ultimately position their devices for successful market entry.
In conclusion, mastering the intricacies of the 510(k) submission process is essential for manufacturers to thrive in a competitive landscape. As the medical device industry continues to evolve, a proactive approach to understanding regulatory requirements and fostering collaboration with experienced professionals will be key to achieving successful outcomes. By prioritizing thorough preparation and strategic planning, manufacturers can navigate this complex terrain with confidence, paving the way for innovation and improved patient care.
Frequently Asked Questions
What is the purpose of the 510k device application?
The 510k device application serves as a premarket notification to the FDA, allowing manufacturers to demonstrate the safety and effectiveness of their medical products that are substantially equivalent to previously marketed devices.
What does 'substantial equivalence' mean in the context of medical devices?
Substantial equivalence refers to the determination that a new product is at least as safe and effective as a previously cleared predicate device. This involves a detailed comparison of the new device's intended use, technological characteristics, and performance data against those of the predicate device.
What role do specialists in Regulatory Affairs play in the 510k submission process?
Specialists in Regulatory Affairs assist manufacturers in navigating the complex regulations surrounding 510k submissions, utilizing their expertise in biomedical engineering and regulatory compliance to ensure all necessary documentation and data are provided for approval.
What is the significance of the DFUF order PIN and PCN in the registration process?
The DFUF order PIN and PCN must be entered after completing registration and device listing, as they are critical for ensuring that the registration is properly recorded and processed.
What are the financial considerations associated with the 510k device process?
Manufacturers must account for fees such as the $6,528 for a 513(g) request for classification information. Additionally, there will be no small business waiver for the annual establishment registration fee for FY 2024, meaning all establishments must pay the same fee.
What has been the trend regarding approval rates for 510k device submissions in 2024?
Approval rates for 510k device submissions in 2024 indicate an increasing efficiency, with many manufacturers successfully navigating the process within an average timeline of 90 days.
What must manufacturers do after obtaining clearance for their medical devices?
After obtaining clearance, manufacturers must register their products with the FDA within 30 days, which includes paying an establishment registration fee for new companies.
Why is understanding substantial equivalence important for manufacturers?
A thorough understanding of substantial equivalence is crucial for manufacturers as it significantly impacts the outcome of the 510k submission process and can facilitate quicker regulatory assessment and market entry.

