Introduction
The 510(k) submission process stands as a pivotal gateway for medical device manufacturers seeking to introduce their innovations to the market. By demonstrating substantial equivalence to existing devices, this premarket notification allows companies to bypass the more rigorous premarket approval pathway, thus expediting their entry into a competitive landscape.
In light of the unique challenges faced by Medtech companies in regions like Latin America, where regulatory complexities and resource fragmentation can hinder progress, a thorough understanding of the 510(k) process becomes paramount.
This article delves into the key components of a successful submission, common pitfalls that lead to rejections, and the critical importance of cybersecurity measures, providing a comprehensive guide for navigating this essential regulatory framework.
What is a 510(k) Submission and Why is it Important?
The 510(k) filing acts as an essential premarket notification to the FDA, intended to show that a medical instrument is significantly comparable to an already marketed product. This pathway is especially crucial for developers, as it allows them to secure FDA clearance and introduce their products to market without the extensive requirements linked to the premarket approval (PMA) procedure. Understanding the nuances of the 510k submission requirements is crucial, as it not only ensures that products meet established safety and effectiveness standards but also enhances their marketability.
In the context of Latin America, where Medtech companies encounter unique challenges—such as regulatory hurdles, language barriers, and fragmented resources—understanding the 510(k) procedure becomes even more essential. The collaboration between Greenlight Guru and bioaccess™ exemplifies efforts to bridge these gaps, facilitating clinical trials that not only accelerate Medtech innovations but also positively impact local economies through job creation and improved healthcare. Their comprehensive clinical trial management services include:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
These services are crucial for navigating the complexities of clinical research in Latin America.
Statistics indicate that a mere 3.2% of approved AI and machine learning (AI/ML)-enabled devices have engaged in clinical trials, predominantly within cardiovascular and radiology specialties. Significantly, the total count of participants in these clinical trials varied from 12 subjects to 2105 subjects, providing a broader perspective on the clinical trial landscape relevant to the 510k submission requirements. The integration of AI technologies, particularly through large language models, is poised to further transform healthcare, promoting equitable access and optimizing resources.
This makes understanding the 510k submission requirements increasingly important in the evolving medical landscape, particularly as highlighted by Mukund Bhandari regarding the balance between innovation and patient safety.
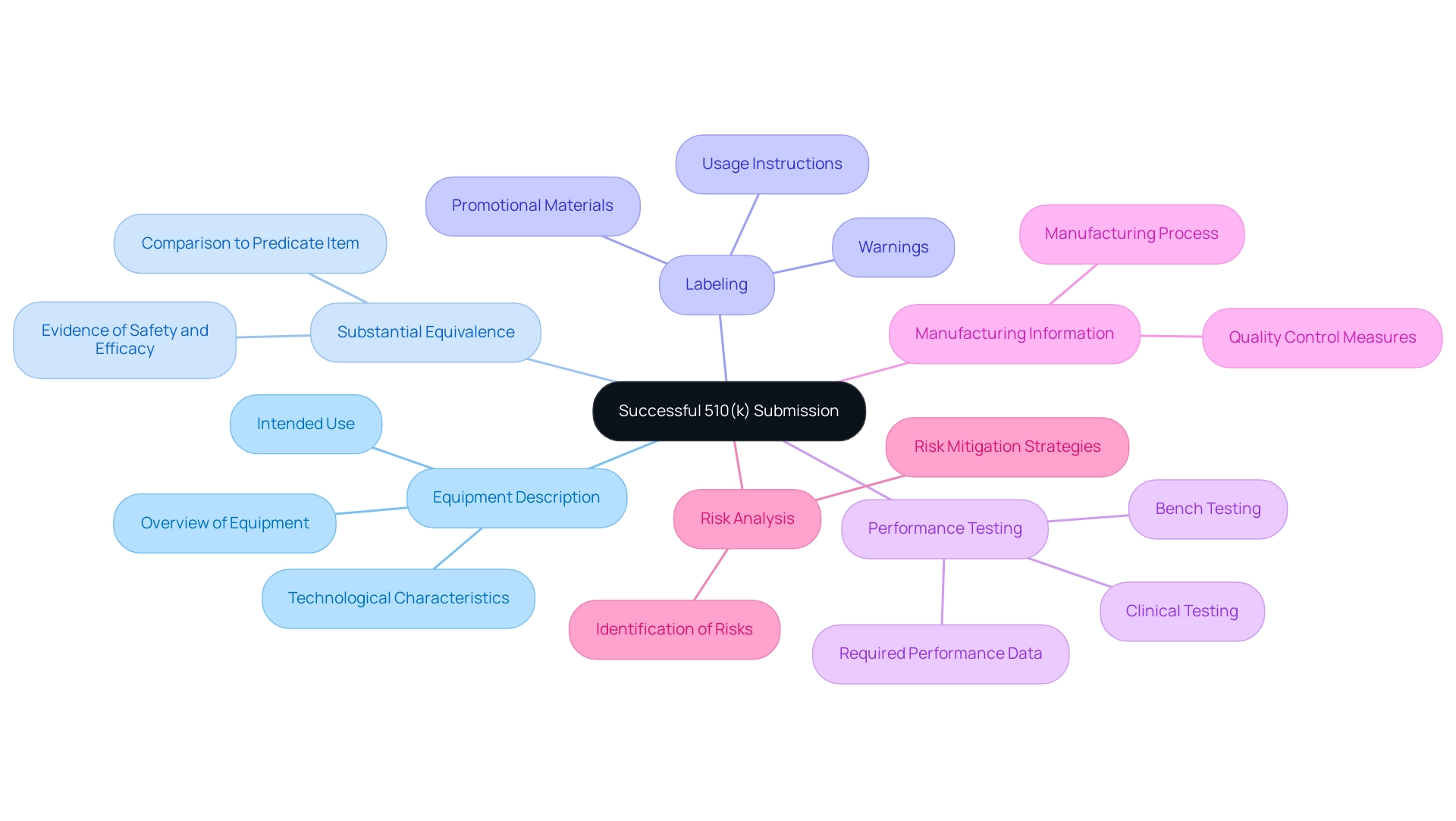
Key Components of a Successful 510(k) Submission
A successful submission requires careful attention to the 510k submission requirements, with several critical components each playing a vital role in the FDA's evaluation process.
- Equipment Description: This section must provide a comprehensive overview of the equipment, detailing its intended use and technological characteristics. A clear and precise description is paramount in aligning with FDA expectations.
- Substantial Equivalence: Applicants must present substantial evidence demonstrating that the item is comparable to a predicate item that is already legally marketed. This comparison is crucial for establishing the new equipment's safety and efficacy.
- Labeling: Proposed labeling should encompass all usage instructions, promotional materials, and necessary warnings. This ensures that end-users are adequately informed about the application's use and potential risks.
- Performance Testing: Submission must include data from required performance, bench, or clinical testing that reinforces the product's safety and effectiveness. This empirical evidence is essential for the FDA's decision-making process.
- Manufacturing Information: Detailed information regarding the manufacturing process and quality control measures must be included. This component assures the FDA of the product's consistent production quality.
- Risk Analysis: A thorough risk assessment should identify potential risks associated with the equipment and outline how these risks are mitigated. This proactive method is essential for tackling safety issues.
Integrating these components efficiently can greatly speed up meeting the 510k submission requirements, thus aiding a successful product launch. The FDA classifies medical instruments into Class I (low risk), Class II (moderate risk), and Class III (high risk), which is essential for understanding the 510k submission requirements.
For U.S. medical equipment firms aiming to maneuver through this procedure in Colombia, collaborating with a trusted CRO like bioaccess® can offer essential assistance.
With over 20 years of expertise in clinical trial management, bioaccess® specializes in Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). Their team, including Regulatory Affairs experts like Ana Criado and Katherine Ruiz, offers comprehensive services that include feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting, ensuring compliance with both local and U.S. regulatory requirements. The importance of bioaccess® as a vetted CRO underscores their capability to assist U.S. medical device companies in successfully navigating the regulatory landscape in Colombia.
Recent advancements, such as the implementation of document conversion and enrichment software like DocShifter, have proven to streamline the complex documentation requirements. A case study on DocShifter demonstrates its effectiveness in greatly accelerating the preparation of PDF documents to meet the 510k submission requirements, thereby improving overall efficiency and facilitating a quicker FDA approval.
Common Reasons for 510(k) Submission Rejections
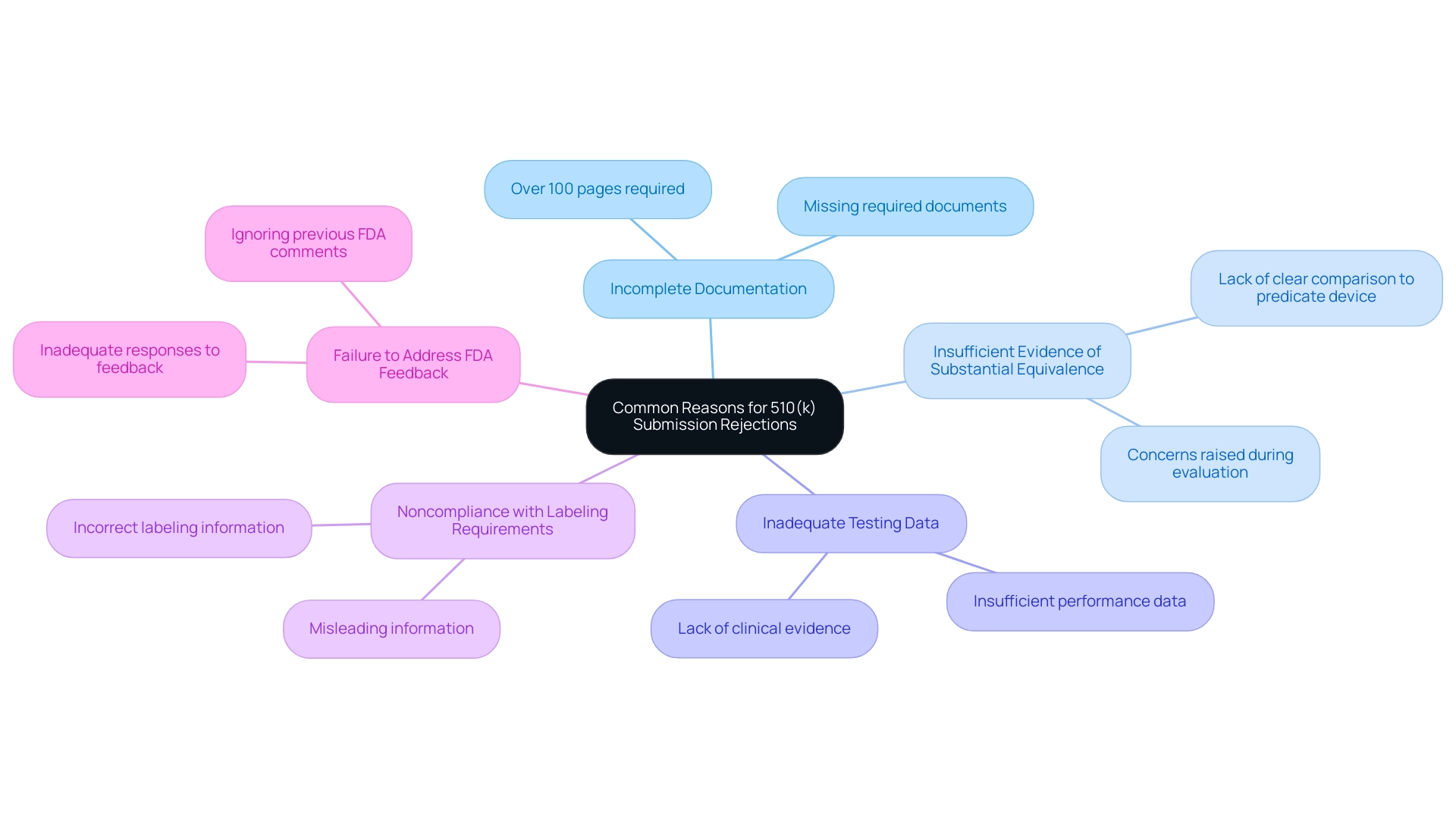
The 510k submission requirements can make the submission procedure challenging, and several factors can lead to rejection. Common reasons include:
- Incomplete Documentation: Most 510(k)s are well over 100 pages; failing to include all required documentation can lead to immediate rejection.
- Insufficient Evidence of Substantial Equivalence: A clear and thorough comparison to a predicate device is crucial. Lacking this can raise significant concerns during the evaluation.
- Inadequate Testing Data: Robust performance data and clinical evidence are essential. Submissions that do not provide sufficient data may be deemed inadequate.
- Noncompliance with Labeling Requirements: Accurate and clear labeling is critical; any incorrect or misleading information can result in rejection.
- Failure to Address FDA Feedback: Ignoring or inadequately responding to previous FDA comments on related proposals can be detrimental, leading to further scrutiny.
As emphasized by the Institute of Medicine,
The Working Group appears to have determined that insufficient or ineffective postmarketing controls warrant heightened scrutiny in the preclearance procedures to safeguard consumers.
This highlights the significance of comprehensive and accurate documentation in fulfilling the 510k submission requirements for 510(k) applications to guarantee safety and effectiveness, especially for Class III products, which face the most stringent examination. Additionally, the case study titled 'Challenges in the 510(k) Clearance Procedure' illustrates the scrutiny encountered by the 510(k) method and recommends reassessing its application for Class III items while highlighting the necessity for enhanced postmarket monitoring.
Moreover, the recent introduction of the Third Party Review Program by the Center for Devices and Radiological Health (CDRH) enables manufacturers to present their 510k submission requirements to acknowledged third parties for evaluation, mirroring current trends in the review. Katherine Ruiz, a Regulatory Affairs expert in medical devices and in vitro diagnostics, can provide invaluable support throughout this process. With her extensive experience in feasibility studies, site selection, compliance reviews, trial setup, and project management, she enhances the likelihood of successful applications by ensuring adherence to the 510k submission requirements.
Katherine holds a degree in industrial microbiology from Universidad Javeriana and a Master's in Quality Management and Integrated Systems from Universidad Santo Tomas, further establishing her authority in navigating the complexities of regulatory requirements.
Navigating Cybersecurity Requirements for 510(k) Submissions
It is essential for developers to adhere to the FDA's guidelines concerning cybersecurity within the 510k submission requirements. The following key points outline the necessary components of a robust cybersecurity strategy:
- Risk Management: Conducting a thorough risk assessment is critical to identifying potential cybersecurity threats that could impact the safety and effectiveness of medical devices.
- Mitigation Strategies: Developers must clearly outline strategies to address the identified risks, ensuring a proactive approach to cybersecurity vulnerabilities.
- Documentation: Comprehensive documentation detailing how cybersecurity issues will be addressed throughout the entire lifecycle of the apparatus is vital. This includes plans for design, development, and ongoing maintenance.
- Post-Market Surveillance: It is equally important for developers to establish plans for monitoring and addressing cybersecurity threats following market entry. This ongoing vigilance is essential to protect patient safety and equipment integrity.
As the landscape of medical equipment continues to evolve, manufacturers are increasingly advised to implement cybersecurity strategies during the design phase. This proactive approach, characterized by the principle of being 'secure by design,' is crucial for ensuring that products are not only effective but also safe from emerging cybersecurity threats. As highlighted by specialists in the field, cybersecurity must be a top priority for all equipment seeking CE Marking, emphasizing the necessity for manufacturers to incorporate these considerations into every aspect of their products.
Furthermore, with the omnibus bill's updates taking effect 90 days after signing, the urgency for compliance is heightened. A case study titled 'Balancing Security and Safety with Effectiveness' illustrates the real-world implications of weak device security, emphasizing that manufacturers must assess security measures in relation to device functionality and intended use.
Step-by-Step Guide to the 510(k) Submission Process
The 510(k) application procedure is a structured pathway that encompasses several critical steps:
- Preparation: Begin by compiling all necessary documentation and data, ensuring that each component is rigorously complete. This foundational step is vital as incomplete entries related to the 510k submission requirements can lead to delays or rejections. Significantly, tests scoring above 12 are categorized as high complexity according to the FDA's scorecard, emphasizing the intricate nature of the application.
- Once preparations are finalized, the completed documentation must be filed in accordance with the 510k submission requirements through the FDA's electronic submission gateway, a streamlined platform that facilitates the review process.
- FDA Review: The FDA will conduct a thorough review of the submission, which may include requests for additional information or clarification. This phase is crucial, as the agency evaluates the safety and effectiveness of the product against the 510k submission requirements of legally marketed counterparts.
- FDA Clearance: If the review is successful, the FDA will issue a clearance letter, authorizing the marketing of the product. This clearance signifies that the apparatus complies with the 510k submission requirements and meets the necessary regulatory standards.
- Post-Clearance Activities: After receiving clearance, it is essential to implement effective post-market surveillance. This involves ongoing monitoring of performance and safety to address any emerging issues, ensuring continued compliance and safeguarding public health.
As stated by the National Human Genome Research Institute, upholding rigorous standards during this procedure is essential for advancing safe and effective medical equipment. Elissa Passiment, EdM, CLS(NCA), Executive Vice President of the American Society for Clinical Laboratory Science, emphasizes the importance of rigorous standards in the approval procedure. Additionally, recent industry shifts have shown that nearly 90% of medical device leaders now prioritize the 510k submission requirements for US regulatory approval over the EU, reflecting a strategic focus on navigating these essential steps efficiently.
Moreover, the skills of professionals such as Katherine Ruiz, a Regulatory Affairs specialist with considerable experience in clinical trials and compliance in Colombia, can greatly improve the efficiency and effectiveness of this application. Katherine specializes in feasibility studies, site selection, compliance reviews, import permits, trial set-up, start-up, and project management, ensuring that all necessary steps are thoroughly managed and documented throughout the 510(k) process. This comprehensive approach not only facilitates smooth submissions in accordance with the 510k submission requirements but also aligns with the regulatory standards essential for market entry.
Conclusion
The 510(k) submission process is fundamental for medical device manufacturers aiming to successfully introduce their innovations to the market. By demonstrating substantial equivalence to existing devices, companies can navigate regulatory challenges more efficiently, particularly in regions like Latin America, where complexities abound. Key components of a successful submission include:
- A thorough device description
- Robust performance data
- Clear labeling
All of which are vital in meeting FDA standards and expediting market entry.
However, the path to approval is fraught with potential pitfalls. Common reasons for submission rejections, such as:
- Incomplete documentation
- Insufficient evidence of substantial equivalence
Highlight the importance of meticulous preparation. Moreover, as the landscape of medical devices evolves, so does the necessity for cybersecurity measures. Manufacturers must adopt a proactive approach to identify and mitigate risks, ensuring the safety and effectiveness of their products.
In conclusion, understanding and effectively navigating the 510(k) submission process is crucial for Medtech companies. By prioritizing comprehensive documentation, adhering to regulatory requirements, and incorporating cybersecurity strategies, manufacturers can enhance their chances of successful submissions. Ultimately, this not only facilitates the introduction of innovative medical devices to the market but also contributes to improved healthcare outcomes and patient safety in an increasingly competitive environment.
Frequently Asked Questions
What is the purpose of the 510(k) filing?
The 510(k) filing serves as a premarket notification to the FDA, demonstrating that a medical instrument is significantly comparable to an already marketed product, allowing developers to secure FDA clearance without the extensive requirements of the premarket approval (PMA) procedure.
Why is understanding the 510(k) submission requirements crucial for developers?
Understanding the 510(k) submission requirements is essential as it ensures that products meet established safety and effectiveness standards, which enhances their marketability.
What unique challenges do Medtech companies face in Latin America regarding the 510(k) process?
Medtech companies in Latin America encounter regulatory hurdles, language barriers, and fragmented resources, making a clear understanding of the 510(k) procedure even more critical.
What services does the collaboration between Greenlight Guru and bioaccess™ provide?
Their collaboration facilitates clinical trials and includes services such as feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting to navigate clinical research complexities in Latin America.
What are the key components required for a successful 510(k) submission?
The key components include: Equipment Description, Substantial Equivalence, Labeling, Performance Testing, Manufacturing Information, and Risk Analysis.
What are common reasons for the rejection of a 510(k) submission?
Common reasons for rejection include: Incomplete Documentation, Insufficient Evidence of Substantial Equivalence, Inadequate Testing Data, Noncompliance with Labeling Requirements, and Failure to Address FDA Feedback.
What is the importance of cybersecurity in the 510(k) submission process?
Cybersecurity is crucial as developers must conduct risk assessments, outline mitigation strategies, document cybersecurity plans, and establish post-market surveillance to protect patient safety and device integrity.
What are the steps involved in the 510(k) application procedure?
The steps include: Preparation of documentation and data, Filing through the FDA's electronic submission gateway, FDA Review of the submission, FDA Clearance if the review is successful, and Post-Clearance Activities for ongoing monitoring and compliance.
How can professionals like Katherine Ruiz assist in the 510(k) submission process?
Katherine Ruiz, a Regulatory Affairs specialist, can enhance the efficiency and effectiveness of the application by managing feasibility studies, compliance reviews, trial setups, and ensuring thorough documentation throughout the 510(k) process.




