Overview
The article highlights five notable clinical trial success stories in Latin America, showcasing the region's growing prominence in medical research due to diverse patient populations, innovative recruitment strategies, and favorable regulatory environments. Key examples include partnerships that significantly reduced recruitment times and improved retention rates, emphasizing the region's capability to effectively conduct trials while also contributing to local economies and healthcare advancements.
Introduction
Latin America is rapidly transforming into a pivotal hub for clinical trials, attracting global attention with its rich diversity of patient populations and supportive regulatory environments. Countries like Brazil, Mexico, and Argentina are at the forefront of this evolution, where increasing numbers of clinical research organizations are setting the stage for groundbreaking studies.
Notably, strategic partnerships, such as the collaboration between bioaccess™ and Caribbean Health Group, are positioning cities like Barranquilla as key players in the clinical research arena. This dynamic shift not only promises enhanced healthcare outcomes but also significantly contributes to local economies through job creation and investment.
As the region embraces innovative patient recruitment strategies and navigates complex regulatory landscapes, it stands poised to make substantial contributions to global health advancements, particularly in critical therapeutic areas.
The Evolving Landscape of Clinical Trials in Latin America
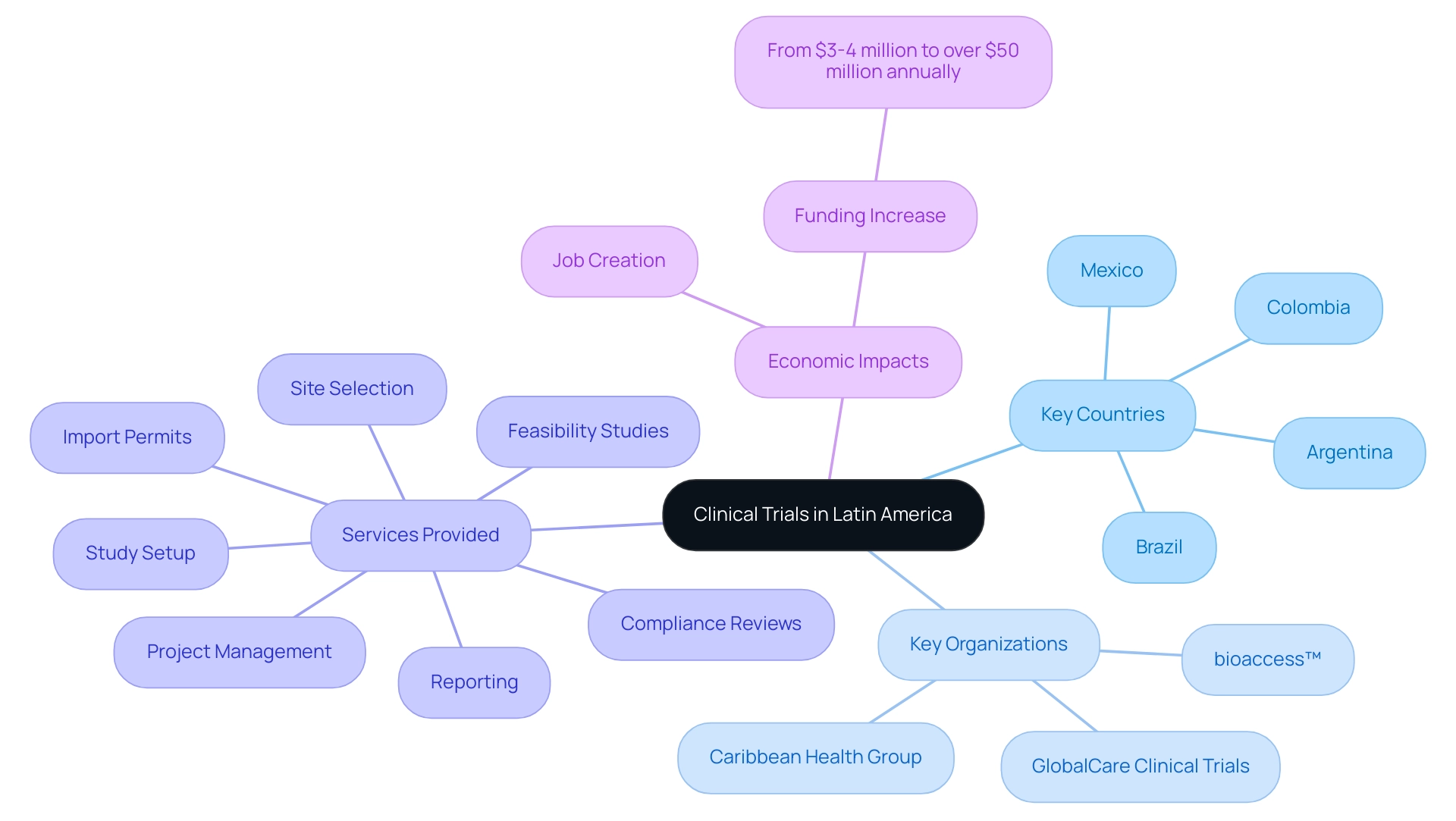
Latin America has emerged as an attractive destination for research studies, driven by its diverse patient populations and favorable regulatory systems, which have led to many Latin America clinical trial success stories. Key participants in this dynamic environment encompass Brazil, Mexico, and Argentina, where the quantity of trial organizations (CROs) is consistently rising. A notable partnership between bioaccess™ and Caribbean Health Group seeks to establish Barranquilla as the premier location for medical studies in the region, backed by Colombia's Minister of Health, Juan Pablo Uribe.
This collaboration is poised to improve local healthcare and draw more research initiatives, further strengthening the region's reputation. Moreover, GlobalCare Clinical Trials has collaborated with bioaccess™ to broaden its services in Colombia, achieving over a 50% decrease in recruitment time and remarkable 95% retention rates, which are key components of Latin America clinical trial success stories through effective research management services including:
- feasibility studies
- site selection
- compliance reviews
- study setup
- import permits
- project management
- reporting
These services guarantee that evaluations meet all essential country requirements, including ethics committee approvals and import permits for investigational devices.
The consequences of Medtech medical studies go beyond investigation, considerably influencing local economies through job creation, economic growth, and enhanced healthcare. For example, the yearly funding in the research sector in the Andean Region of South America has risen from $3-4 million to over $50 million annually, emphasizing the area's growing importance in the worldwide medical research arena. As a result, the region is witnessing a significant increase in research activity, especially in therapeutic fields like oncology, cardiology, and infectious diseases, contributing to Latin America clinical trial success stories and highlighting the area's potential.
Spotlight on Women in STEM: Pioneering Clinical Trials
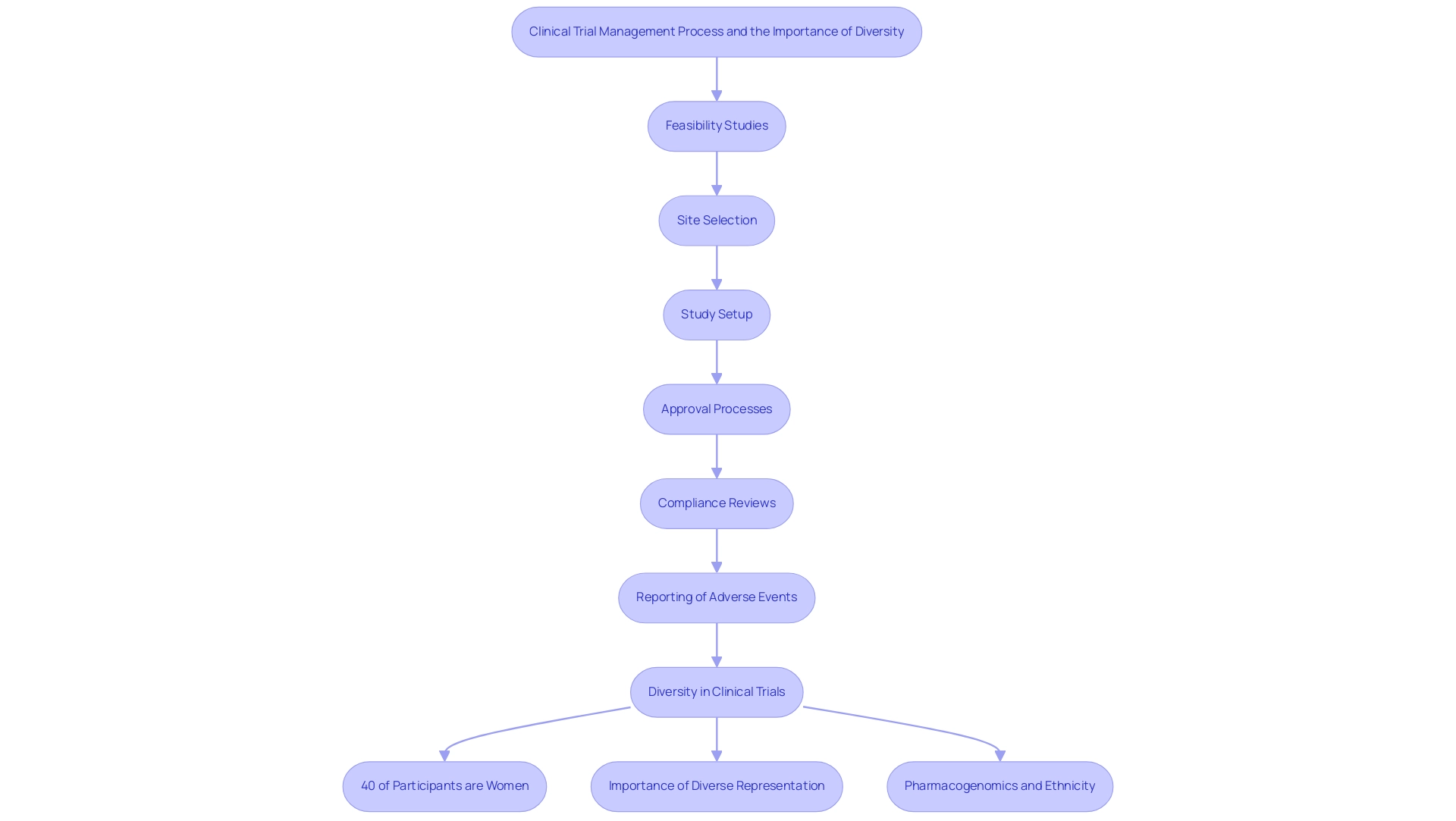
Dr. Maria Elena Bottazzi stands out as an exceptional example among Latin America clinical trial success stories in the field of vaccine development and research. As a co-director of the Texas Children's Center for Vaccine Development, she has spearheaded pivotal research focused on vaccines for neglected tropical diseases. Her work exemplifies the comprehensive research study management services essential for success in this field, including:
- Feasibility studies
- Site selection
- Study setup
- Approval processes from ethics committees and health ministries
- Compliance reviews
- Reporting of serious and non-serious adverse events
This is especially important in areas dealing with healthcare disparities, where such studies can result in job creation, economic growth, and healthcare enhancements. Significantly, as of 2019, women made up approximately 40% of participants in medical studies for cancer, cardiovascular disease, and psychiatric disorders, despite constituting 51% of the U.S. population. This statistic underscores the critical need for inclusive practices in clinical trials.
Dr. Bottazzi's dedication to advancing diversity is essential, as it not only encourages innovative studies but also acts as a motivation for young women aiming to enter STEM fields. Maria Brooks, a PhD professor of epidemiology and biostatistics, expresses hope in the academic community's dedication to enrolling and retaining a broader group of participants, further emphasizing the importance of diversity. Furthermore, studies have indicated that cardiovascular medications can have varying effects depending on ancestry, emphasizing the importance of diverse representation in medical trials.
Media coverage by Clinical Leader concerning clinical studies in America and Colombia further amplifies the significance of Dr. Bottazzi's leadership, showcasing her role in creating Latin America clinical trial success stories that exemplify the transformative impact female scientists can have on clinical study outcomes. Furthermore, the processes of obtaining import permits and nationalizing investigational devices are critical components of the management framework, reinforcing the ongoing need for inclusive practices that engage a broader spectrum of research participants.
Innovative Patient Recruitment Strategies in Latin America
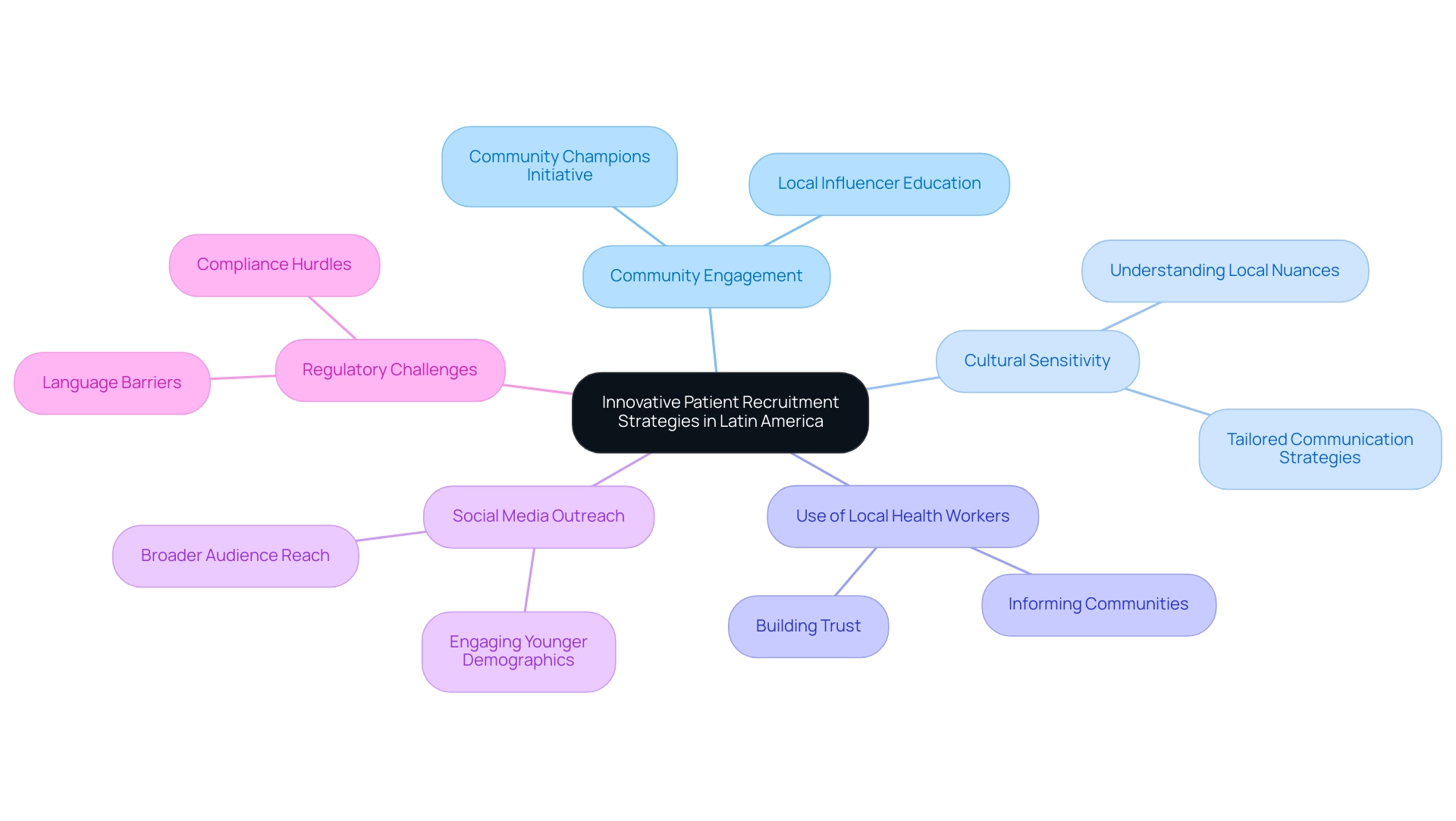
In Latin America, innovative patient recruitment strategies are increasingly prioritizing community engagement and culturally sensitive approaches. Employing local health workers to inform communities about medical studies has shown considerable effectiveness in boosting participation rates. This method not only builds trust but also ensures that information is conveyed in a manner that resonates with local populations.
Moreover, as highlighted by industry leaders like Steve Garchow, understanding the unique healthcare systems and cultural nuances is crucial for success in this region. Medtech companies face challenges such as regulatory hurdles and language barriers, which can complicate the recruitment process. The rise of social media platforms and mobile health technologies has enabled researchers to reach a broader audience, particularly among younger demographics, who are often more engaged online.
A significant success story that contributes to Latin America clinical trial success stories is Brazil's 'Community Champions' initiative, which educates local influencers to promote research studies. This grassroots approach has led to remarkable improvements in both enrollment and retention rates, highlighting the importance of Latin America clinical trial success stories in developing a proactive market access strategy. By implementing these strategies and fostering collaboration with local stakeholders, clinical studies are becoming more representative of the populations they aim to serve, ultimately promoting a more inclusive research landscape.
As Homedes and Ugalde noted, "in Latin America, there are researchers who recruit their own patients, even to the point of reviewing medical records in public facilities to identify possible participants." This active method of recruitment is vital in tackling the unique health challenges encountered by local communities, ensuring that the studies conducted are not only relevant but also advantageous to the population. Considering that pharmaceutical firms assert it requires an average of 10 to 15 years and $2.6 billion to deliver a new medication to patients, effective patient recruitment strategies are crucial for enhancing the efficiency of research studies.
Furthermore, obtaining worldwide market data and business profiles via a complimentary account can offer significant understanding of the environment of medical studies in South America.
Navigating the Regulatory Landscape for Successful Trials
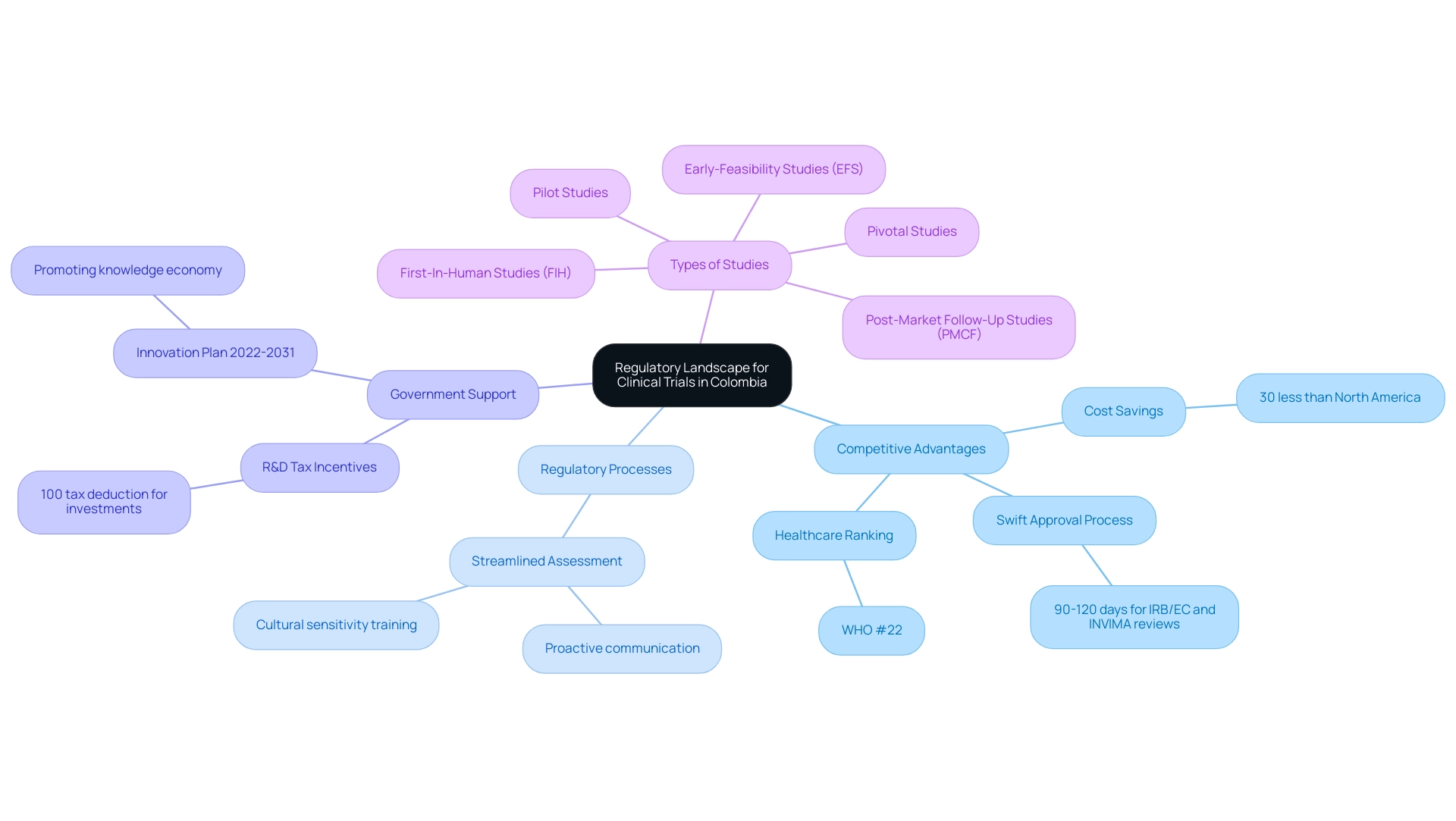
Recent changes in the regulatory environment for research studies throughout Latin America have resulted in significant advancements that contribute to Latin America clinical trial success stories, especially in Colombia. The nation distinctly stands out because of its competitive advantages for first-in-human (FIH) trials, including:
- Substantial cost savings of over 30% compared to trials in North America and Western Europe
- A relatively swift approval process with IRB/EC and INVIMA reviews taking only 90-120 days
Significantly, Colombia's healthcare system has gained international recognition, being ranked #22 by the World Health Organization and among the top five worldwide by International Living, which increases its attractiveness for performing research studies.
Furthermore, the Colombian government offers substantial R&D tax incentives, including a 100% tax deduction for investments in science, technology, and innovation projects, which can significantly benefit medical device companies.
bioaccess® offers accelerated medical device research study services in Colombia, leveraging over 20 years of expertise in managing:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies (PMCF)
This customized strategy establishes bioaccess® as a reputable contract study organization (CRO) and consulting ally for U.S. medical device firms aiming to achieve Latin America clinical trial success stories while navigating the Colombian trial environment successfully. To address challenges related to ethics committee delays, bioaccess® employs strategies such as proactive communication and cultural sensitivity training, ensuring informed consent processes are streamlined and effective.
While Brazil's National Health Surveillance Agency (ANVISA) has instituted measures to streamline its review process, Colombia has also recognized the importance of reducing bureaucratic hurdles and enhancing international collaboration. By investing in science, technology, and innovation, the Colombian government aims to support job creation, economic growth, and healthcare advancements, establishing a robust environment for medical research. Furthermore, with a population exceeding 50 million and approximately 95% coverage by universal healthcare, patient recruitment in Colombia offers a considerable benefit for research studies.
As Julio G. Martinez-Clark, CEO of bioaccess®, emphasizes, Colombia's ambitious innovation plan for 2022–2031 establishes it as a key participant in the global medtech arena, promoting trust and adherence in research execution.
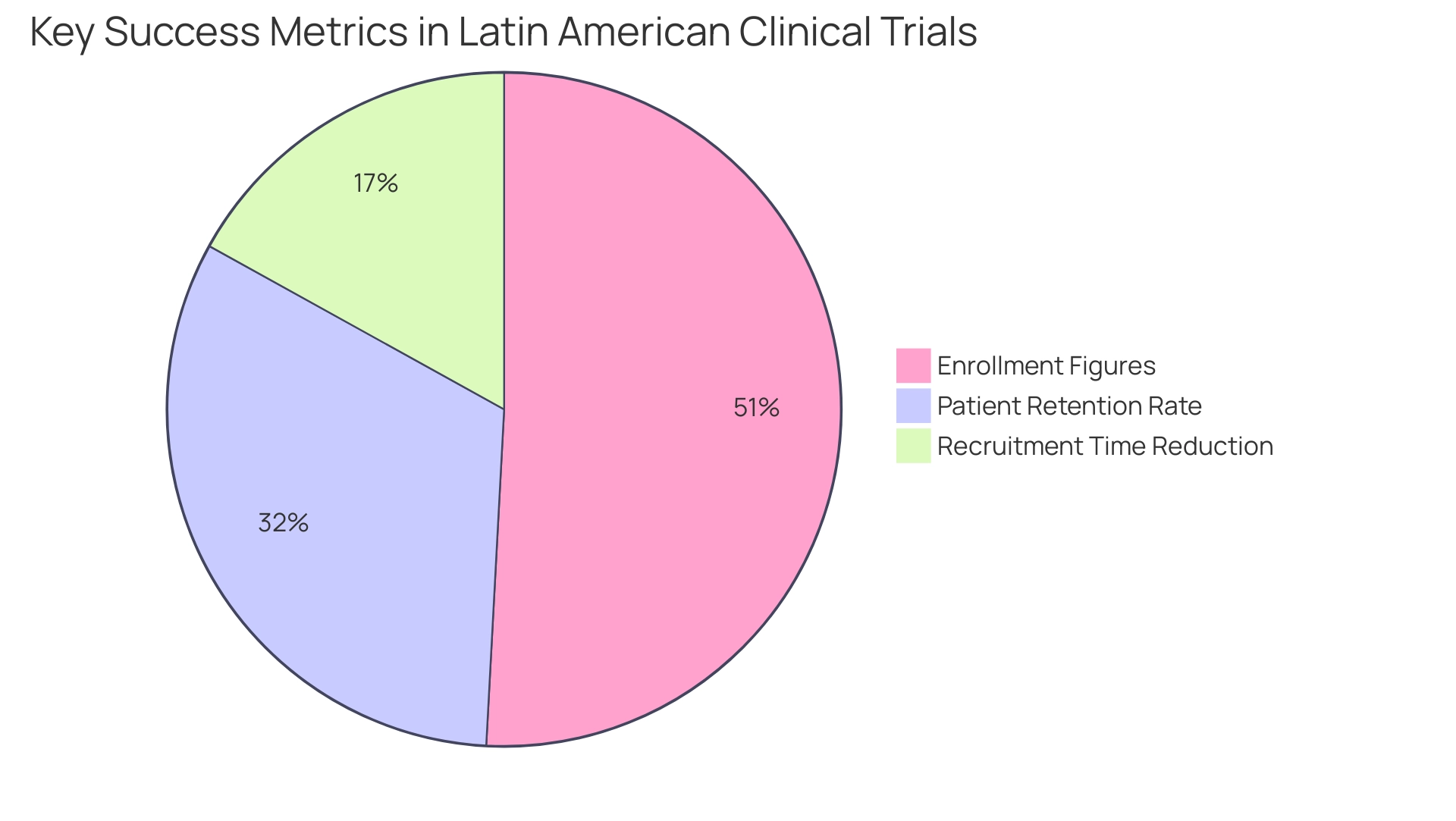
Key Success Metrics from Latin American Clinical Trials
Key success indicators from medical studies contribute to Latin America clinical trial success stories, highlighting the region's growing impact in global research. A significant instance that highlights Latin America clinical trial success stories is the partnership between GlobalCare Clinical Trials and bioaccess™ to improve ambulatory services for research in Colombia, which accomplished over a 50% decrease in recruitment time and an impressive 95% patient retention rate. Such high retention rates are indicative of effective patient engagement strategies within the region.
Furthermore, trials targeting rare diseases have witnessed exceptional enrollment figures, with some reporting participation rates exceeding 150% of their initial targets. These statistics not only demonstrate the effectiveness of innovative recruitment methods but also establish South America as a promising center for Latin America clinical trial success stories. Investigators in Latin America contribute an average of 7 years of overall experience in medical studies, further enhancing the region's capability.
As Mirella Nardo from ICESP highlights, the expected enhancements from upcoming legislation in Brazil (PL 7082/2017) could further promote healthcare practices, improving access to new therapies and establishing elevated standards. This legislation symbolizes hope for better access to new cancer therapies and enhanced study standards. Furthermore, Argentina is anticipated to record the highest CAGR in the Latin America research market from 2024 to 2030, mirroring broader trends in the region's research landscape.
By sharing the Latin America clinical trial success stories, including media coverage from Clinical Leader, the region can foster confidence among stakeholders and stimulate greater investment in its clinical trial landscape.
Conclusion
Latin America is rapidly becoming a significant hub for clinical trials, characterized by its diverse patient populations and supportive regulatory frameworks. Key players like Brazil, Mexico, and Argentina are leading this transformation, notably through partnerships such as bioaccess™ and Caribbean Health Group in Barranquilla. These collaborations not only enhance healthcare outcomes but also stimulate local economies through job creation and increased investment.
Innovative patient recruitment strategies that emphasize community engagement and cultural sensitivity are crucial for addressing local health challenges. Impressive success metrics, including reduced recruitment times and high retention rates, demonstrate the region’s capability for impactful research.
As regulatory environments improve, particularly in Colombia, which offers competitive advantages for clinical trials, Latin America is poised to become a leader in global clinical research. A focus on diversity and inclusion further strengthens this potential, ensuring that research reflects the populations it serves.
In conclusion, Latin America’s evolution into a key player in clinical trials signifies important advancements in healthcare and presents opportunities for inclusive and innovative research. This shift is set to influence global health outcomes, making engagement and investment in the region essential for stakeholders.
Frequently Asked Questions
Why has Latin America become an attractive destination for clinical research studies?
Latin America has emerged as an appealing location for research studies due to its diverse patient populations and favorable regulatory systems, leading to numerous clinical trial success stories.
Which countries in Latin America are key participants in clinical trials?
Key participants in clinical trials in Latin America include Brazil, Mexico, and Argentina, where the number of trial organizations (CROs) is consistently increasing.
What is the significance of the partnership between bioaccess™ and Caribbean Health Group?
The partnership aims to establish Barranquilla as the premier location for medical studies in the region, supported by Colombia's Minister of Health, Juan Pablo Uribe. This collaboration is expected to enhance local healthcare and attract more research initiatives.
What improvements have GlobalCare Clinical Trials achieved through its collaboration with bioaccess™ in Colombia?
GlobalCare Clinical Trials has achieved over a 50% decrease in recruitment time and a remarkable 95% retention rate, which are essential components of successful clinical trials.
What services are provided to ensure successful clinical trials in Latin America?
Key research management services include feasibility studies, site selection, compliance reviews, study setup, import permits, project management, and reporting, ensuring that evaluations meet essential country requirements.
How do Medtech medical studies impact local economies in Latin America?
Medtech studies significantly influence local economies by creating jobs, fostering economic growth, and enhancing healthcare quality.
What has been the trend in research sector funding in the Andean Region of South America?
Funding in the research sector has increased from $3-4 million to over $50 million annually, highlighting the region's growing importance in global medical research.
In which therapeutic fields is research activity significantly increasing in Latin America?
Research activity is notably increasing in therapeutic fields such as oncology, cardiology, and infectious diseases.
Who is Dr. Maria Elena Bottazzi, and what is her contribution to clinical trials in Latin America?
Dr. Maria Elena Bottazzi is a co-director at the Texas Children’s Center for Vaccine Development, recognized for her pivotal research on vaccines for neglected tropical diseases, exemplifying successful clinical trial management.
What is the importance of diversity in clinical trials, as highlighted in the article?
Diversity in clinical trials is crucial as it can lead to innovative studies, better representation of various populations, and more effective treatments, especially since cardiovascular medications can have varying effects based on ancestry.
What role does media coverage play in the recognition of clinical trial success stories?
Media coverage, such as that from Clinical Leader, highlights the significance of leaders like Dr. Bottazzi in creating success stories, showcasing the transformative impact of female scientists on clinical study outcomes.

