Overview
Latin America presents significant medical research opportunities due to its diverse populations, growing research institutions, and increasing investments in healthcare innovation. The article highlights that countries like Brazil, Mexico, and Argentina are at the forefront of this evolution, although challenges such as regulatory hurdles and language barriers must be addressed to foster effective collaborations and maximize the region's potential in global health research.
Introduction
Latin America is rapidly establishing itself as a pivotal player in the realm of medical research, characterized by its unique demographic diversity and a burgeoning network of clinical research institutions. This transformation is fueled by substantial investments in healthcare innovation, evidenced by a remarkable growth trajectory in the clinical trials matching software market.
With projections indicating a significant increase in revenue by 2030, the region is poised to become a strategic destination for rigorous clinical studies aimed at addressing both local health challenges and global health issues.
However, navigating the complexities of regulatory frameworks and fostering effective collaborations remains essential for maximizing the potential of this emerging landscape. As the region continues to evolve, understanding its advantages, challenges, and future directions will be crucial for stakeholders aiming to harness its capabilities in advancing medical science.
The Landscape of Medical Research in Latin America
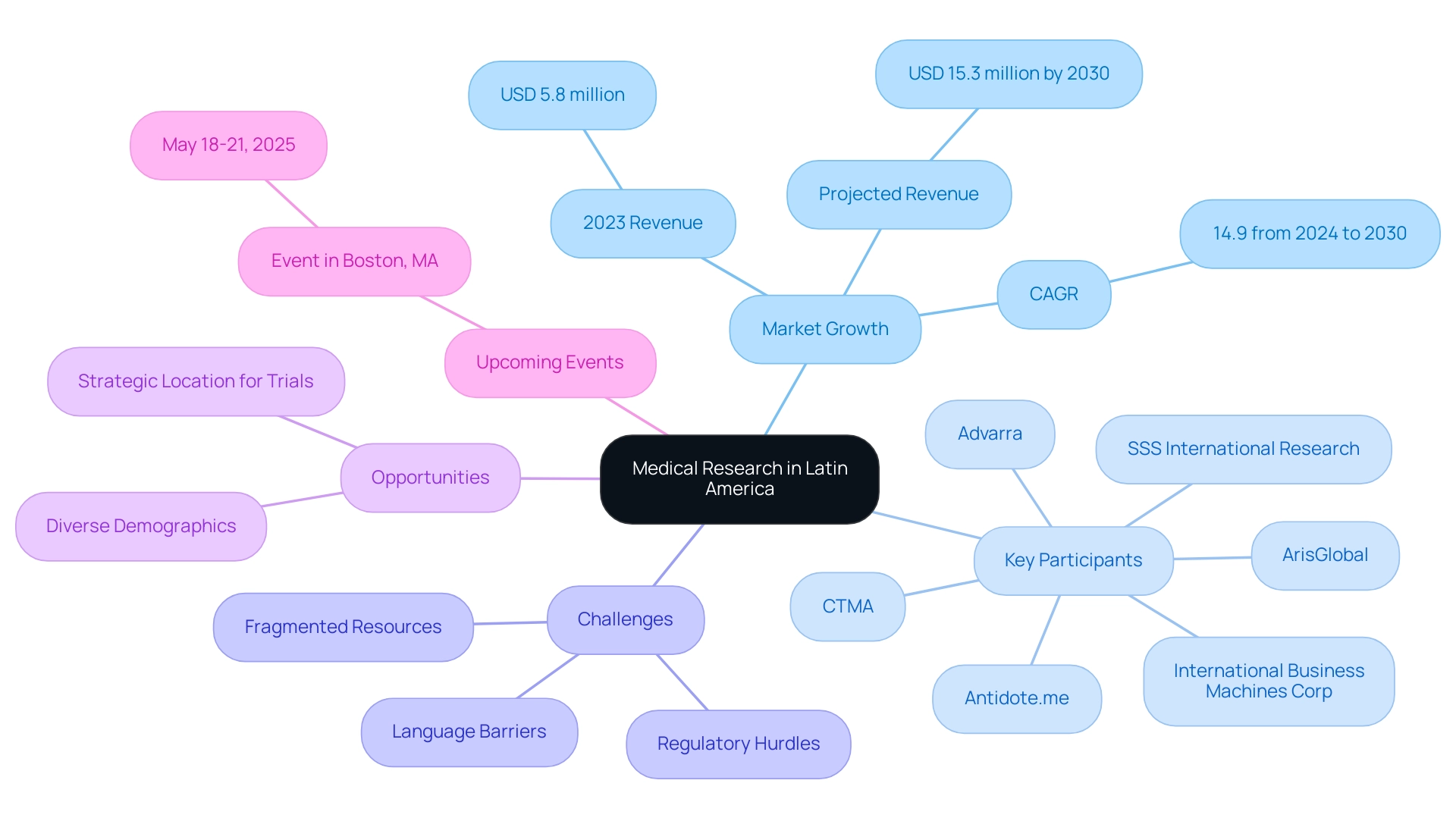
The region of Latin America is rising as a dynamic center for Latin America medical research opportunities, characterized by its varied populations and a consistently growing number of research institutions. This evolution is underscored by significant investments in healthcare innovation across the region. Significantly, the research matching software market in Latin America produced a revenue of USD 5.8 million in 2023, with forecasts suggesting an increase to USD 15.3 million by 2030, indicating a compound annual growth rate (CAGR) of 14.9% from 2024 to 2030.
Such growth is indicative of the region's expanding capacity for conducting thorough research studies. Key market participants, including:
- International Business Machines Corp
- ArisGlobal
- Antidote.me
- CTMA
- SSS International Research
- Advarra
are actively contributing to this landscape, offering comprehensive management services such as:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting
Countries such as Brazil, Mexico, and Argentina are leading the charge in creating Latin America medical research opportunities, hosting a multitude of studies aimed at addressing both regional health challenges and broader global health issues.
However, US Medtech companies face challenges such as:
- Regulatory hurdles
- Language barriers
- Fragmented resources
which hinder effective collaboration with Latin American hospitals. Addressing these challenges is crucial for enhancing communication and fostering partnerships. As emphasized by partnerships like the one between Greenlight Guru and bioaccess™ to accelerate Medtech innovations, and demonstrated by PAVmed's first in-human study in Colombia, there are significant Latin America medical research opportunities that position the region as a strategic location for conducting trials.
This provides access to a rich demographic diversity that enhances the relevance and applicability of findings. Furthermore, a significant event associated with this subject will occur from May 18, 2025, to May 21, 2025, in Boston, MA, emphasizing ongoing involvement and advancements in the area of medical studies.
Advantages of Conducting Clinical Trials in Latin America
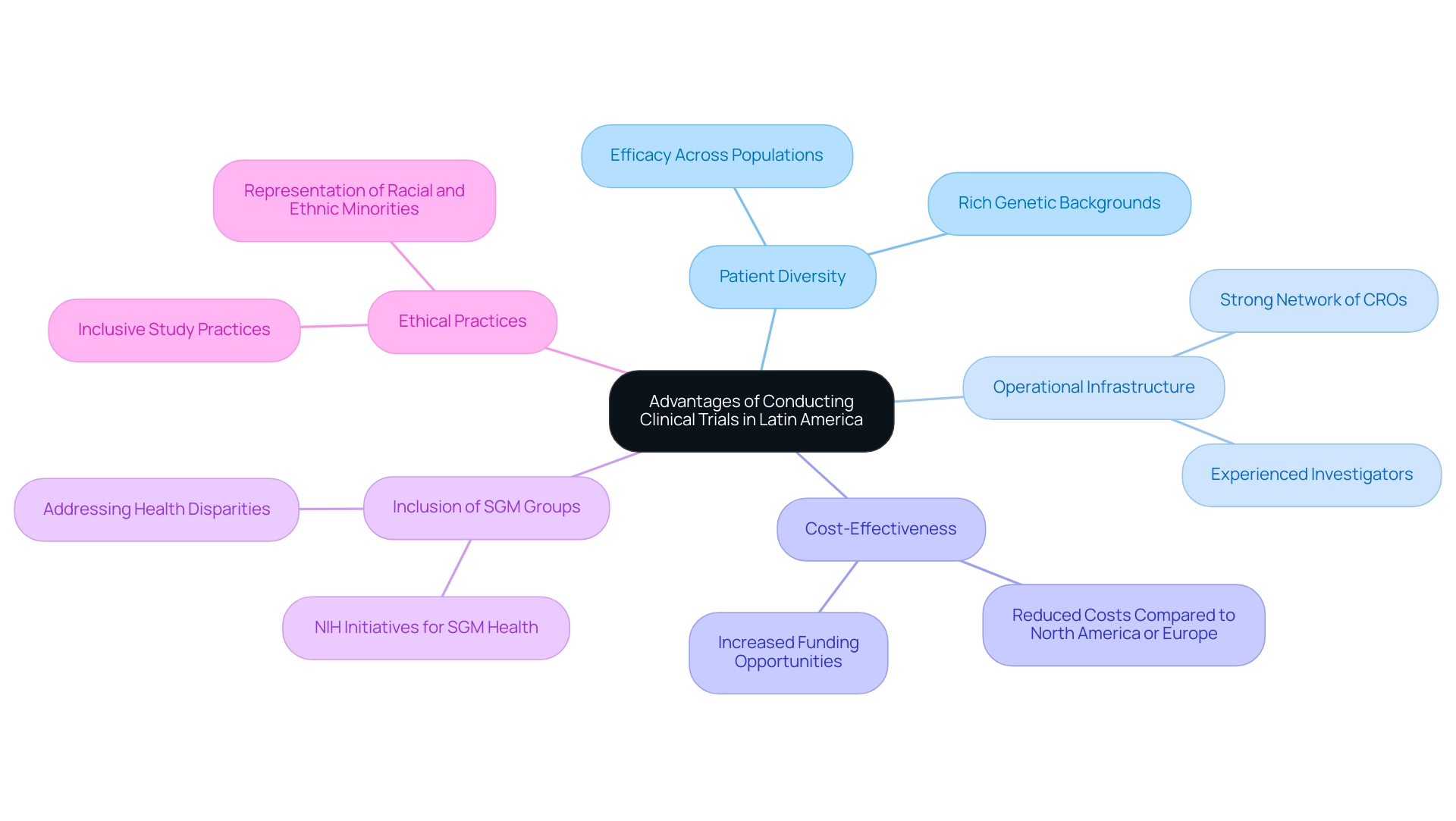
Carrying out medical trials in South America highlights the various benefits associated with Latin America medical research opportunities, which are progressively acknowledged in the medical community. A primary benefit is the region's rich patient diversity, encompassing a wide range of genetic backgrounds and disease manifestations. This diversity is instrumental in developing treatments demonstrating efficacy across heterogeneous populations, making findings more applicable globally.
For instance, ReGelTec's Early Feasibility Study on HYDRAFIL™ involved eleven patients in Colombia, successfully treated for chronic low back pain. The innovative approach utilized a patented hydrogel, which is melted before being injected into the nucleus of a degenerated disc via a 17-gauge needle, showcasing the potential for advanced therapies in diverse settings.
The operational infrastructure in many Latin American countries is also noteworthy. A strong network of research organizations (CROs) and seasoned investigators ensures studies are facilitated efficiently and effectively.
Significantly, GlobalCare Clinical Studies has collaborated with bioaccess™ to improve ambulatory services in Colombia, achieving over a 50% reduction in recruitment duration and 95% retention rates. This operational efficiency, along with cost-effectiveness, increases the attractiveness of performing research studies in this region. Studies suggest that the total costs related to medical studies in Latin America can be significantly reduced compared to those in North America or Europe, without compromising the quality of the investigation.
This financial benefit is essential for numerous organizations seeking to enhance their funding for studies while still participating in thorough investigations.
Moreover, as per the NIH's strategic plan, boosting the representation of sexual and gender minority groups in medical studies is important. The NIH emphasizes that SGM individuals are a health-disparities population and highlights the need for a strong workforce to support this initiative, thus underscoring the importance of addressing health disparities within these communities and enhancing patient diversity.
As noted in the report 'Trends in Women's Representation in Research Studies,' while there has been progress in including women in research, significant gaps remain, particularly for racial and ethnic minority women. This suggests a substantial opportunity for Latin America medical research opportunities to lead in offering inclusive study practices that represent the diversity of the populations being examined. Such initiatives not only promote ethical investigative practices but also enhance the overall quality and significance of clinical studies, ultimately benefiting the global community.
Dushyanth Surakanti, Founder and CEO of Sparta Biomedical, remarked, 'The collaboration with bioaccess™ has significantly improved our testing processes, allowing us to concentrate on innovation while ensuring patient care remains paramount.
Challenges and Barriers in Latin American Medical Research
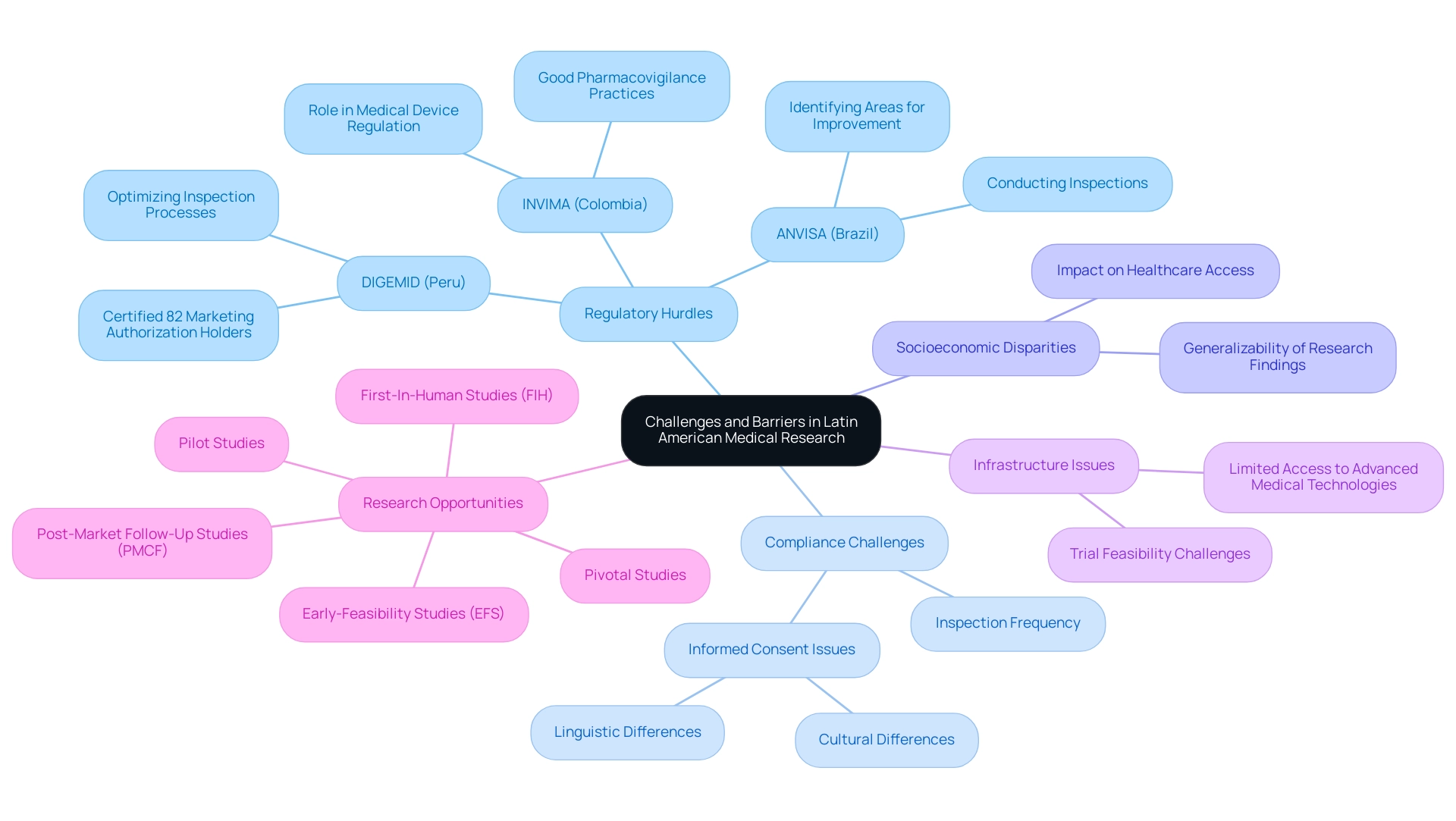
Navigating the unique set of challenges presented by Latin America medical research opportunities requires careful attention. Regulatory hurdles vary significantly across countries, creating a complex landscape for study approvals and compliance adherence. In Colombia, for instance, the National Food and Drug Surveillance Institute (INVIMA) plays a crucial role as a Level 4 health authority, overseeing medical device regulation and ensuring compliance with good pharmacovigilance practices.
Regulatory authorities like DIGEMID in Peru and ANVISA in Brazil have emphasized the need for robust compliance, conducting numerous inspections to ensure standards are met. DIGEMID has certified 82 marketing authorization holders and is optimizing its inspection processes, while ANVISA continues to identify areas for improvement. These regulatory challenges can postpone the commencement of clinical studies, particularly when ethics committees encounter problems with informed consent—a situation worsened by linguistic and cultural differences.
Additionally, limited access to advanced medical technologies in certain regions creates infrastructure issues impacting trial feasibility. Socioeconomic disparities across populations also play a critical role, often affecting healthcare access and the generalizability of research findings. Notably, dropout rates in Latin America are one-third of those in the U.S. and the EU, posing challenges in participant retention.
Experts highlight that addressing these multifaceted challenges is essential; successful execution of studies hinges on careful planning and collaboration with local experts who understand the regional landscape. With bioaccess®'s expertise in comprehensive research management, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies (PMCF)
organizations can navigate these hurdles effectively. Furthermore, bioaccess® provides essential services such as feasibility studies, site selection, regulatory compliance, setup for testing, project management, and reporting.
With 52% of global clinical trials taking place outside the U.S., America is increasingly emerging as a preferred center for trials. As the landscape for medical investigation evolves, particularly looking toward 2024, understanding these regulatory hurdles will be crucial for fostering effective US collaborations that leverage Latin America medical research opportunities in the medtech sector. Argentina is expected to register the highest CAGR from 2024 to 2030, further underscoring the growth potential in the region.
Emerging Trends and Future Directions in Latin American Medical Research
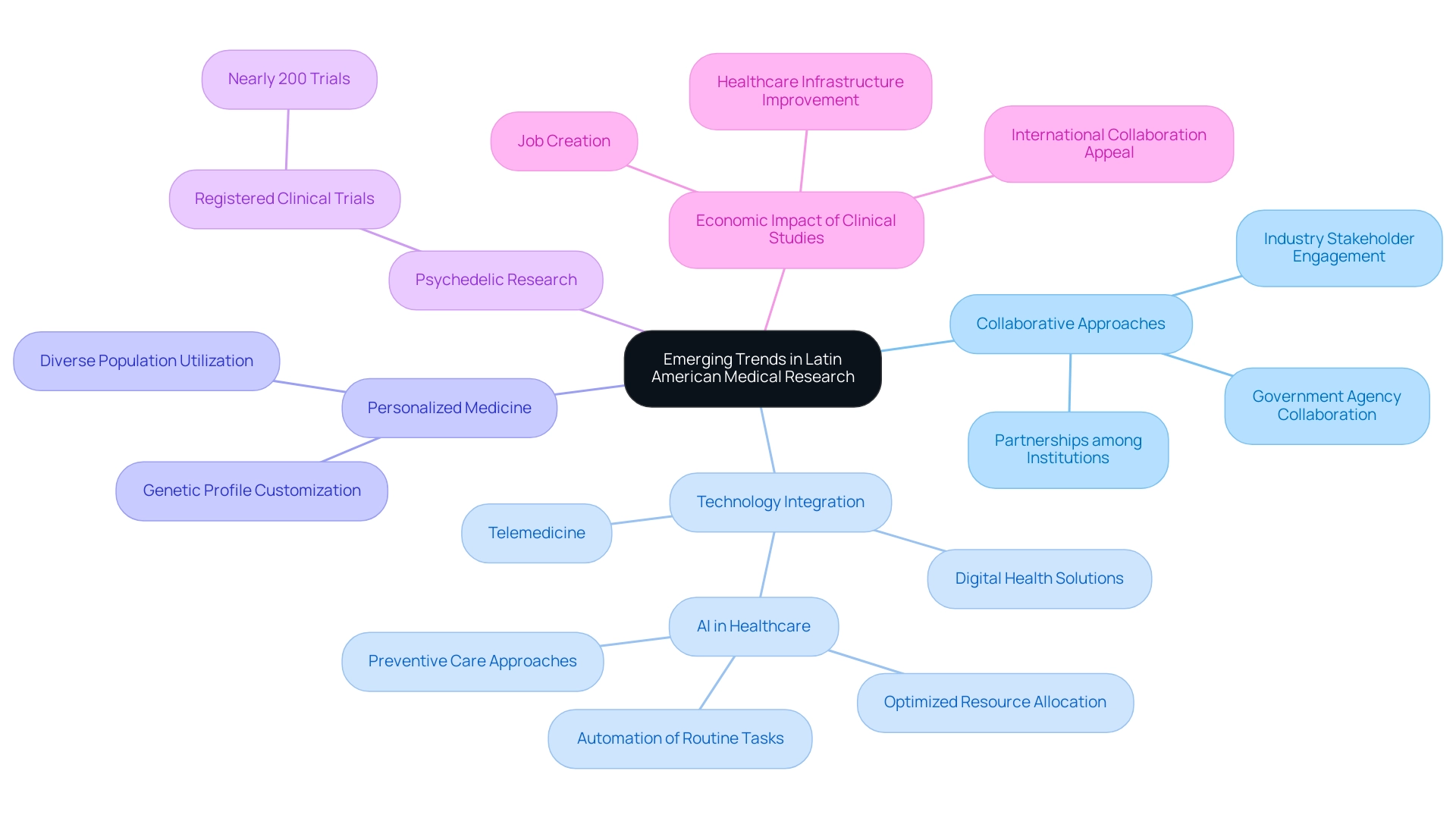
Emerging trends in Latin America medical research opportunities reveal a significant shift towards collaborative and multidisciplinary approaches. This evolution is prominently characterized by the integration of technology into investigation methodologies, particularly through the utilization of telemedicine and digital health solutions. The COVID-19 pandemic has accelerated this trend, highlighting the necessity for adaptable healthcare delivery systems.
Furthermore, there is a notable emphasis on personalized medicine, which customizes treatment based on individual genetic profiles, capitalizing on the region's diverse populations. The expansion of medical studies, featuring almost 200 registered investigations centered on psychedelics, signifies a groundbreaking field of inquiry gaining momentum in the region. Additionally, with the expertise of bioaccess® in managing comprehensive clinical trial services, including:
- feasibility studies
- site selection
- compliance reviews
- trial setup
- import permits
- project management
- reporting
- the nationalization of investigational devices
the potential for impactful collaboration is vast.
Clinical studies significantly contribute to local economies by creating jobs and improving healthcare infrastructure, further enhancing the region's appeal for international collaboration. As Dr. Acosta from the Rajpurkar Lab notes, 'AI also offers significant potential for reducing healthcare costs through optimized resource allocation, automation of routine tasks, and enabling preventive care approaches.' The pressure to improve medical technology and delivery infrastructure has been reported by two-thirds (69%) of insurers as a top driver of medical costs, particularly in the Americas (88%).
This underscores the need for sustainable healthcare solutions. Looking forward, strengthening partnerships among academic institutions, industry stakeholders, and government agencies is crucial for enhancing capacity and fostering innovation. These developments not only indicate a promising future for Latin America medical research opportunities but also establish the region as a crucial contributor to global health progress.
The 2025 WTW Global Medical Trends Survey indicates that a substantial portion of data—25%—originates from the Americas, with 35% from Asia Pacific and 15% from the Middle East and Africa, underscoring the significance of this region in the broader context of medical studies.
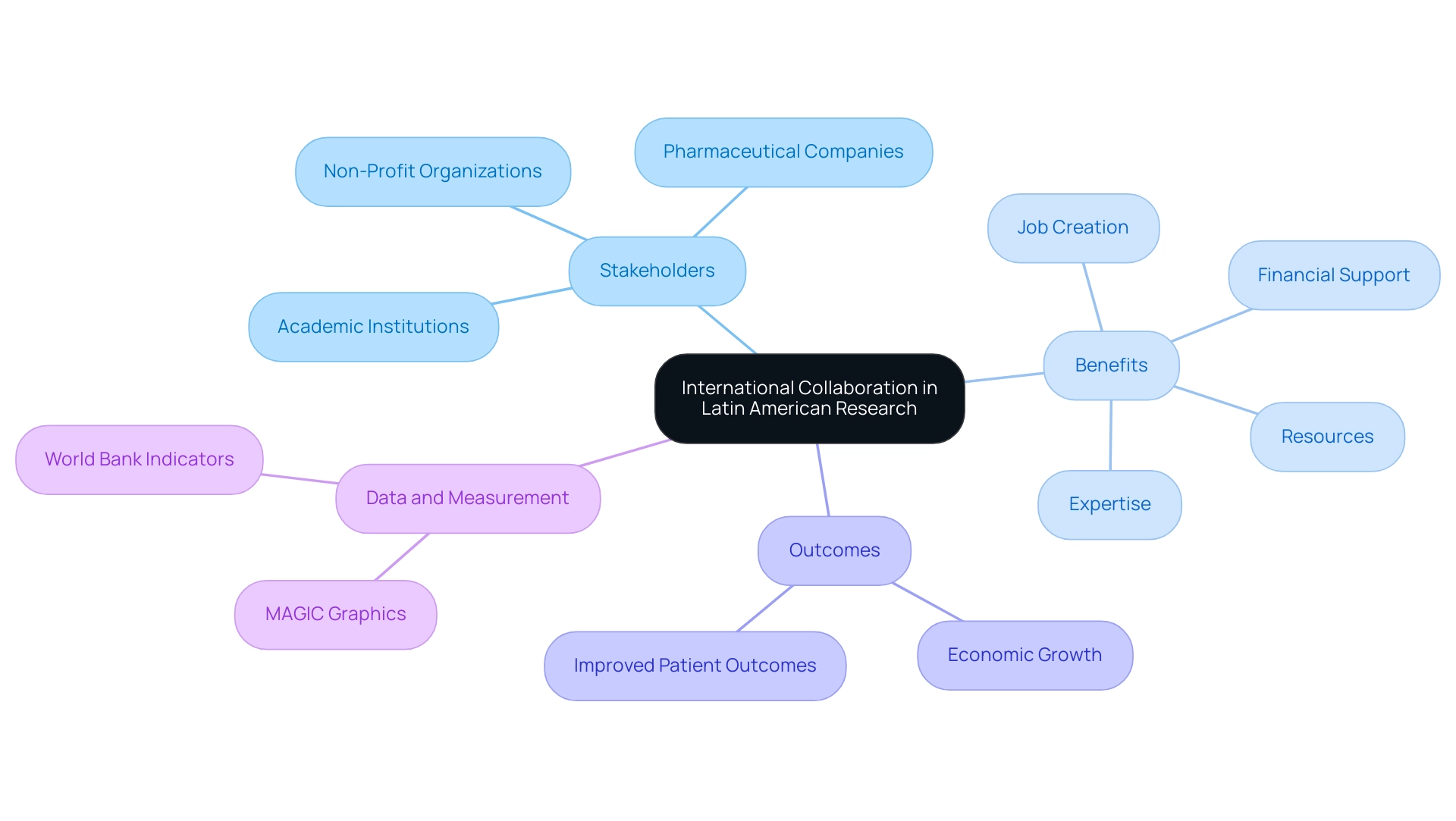
The Role of International Collaboration in Advancing Latin American Research
International collaboration is crucial for advancing Latin America medical research opportunities, as it provides a pathway to improved quality and scope through partnerships with global academic institutions, pharmaceutical companies, and non-profit organizations. These collaborations provide critical resources, expert knowledge, and financial support that can enhance local capabilities, fostering job creation and economic growth. Significantly, leaders such as Julio Martinez-Clark, CEO of bioaccess, support Medtech clinical studies in the region, operationalizing clinical trials that not only create innovative therapies for local health challenges but also greatly enhance patient outcomes.
Such partnerships facilitate knowledge transfer and capacity building within local institutions, enabling them to thrive in a competitive academic landscape. Furthermore, the emotional impact of these advancements cannot be overstated, as they transform lives and communities, contributing to international recognition for local economies. The World Bank's World Development Indicators (WDI) serves as a crucial resource, offering statistical data on over 550 development indicators from 1960 to 2003 for more than 200 countries, thus providing an evidence-based context for understanding the socio-economic environment in which these collaborations occur.
This data is vital for identifying trends and measuring the impact of international partnerships on local health outcomes. Additionally, MAGIC provides an interactive graphic system for international trade data, which can be instrumental in visualizing the flow of resources and knowledge across borders, further emphasizing the importance of these collaborations. By sharing best practices and aligning standards, international partnerships can streamline regulatory processes, ultimately speeding up the pace of investigation.
As Latin America strengthens its research capabilities, the cultivation of global partnerships will be imperative for success in Latin America medical research opportunities.
Conclusion
The landscape of medical research in Latin America is rapidly evolving, showcasing the region's potential as a key player in global healthcare innovation. The unique demographic diversity and the robust network of clinical research institutions are significant assets, supported by substantial investments in healthcare technology and infrastructure. The projected growth of the clinical trials matching software market underscores the region's expanding capacity for conducting rigorous clinical studies, which are essential for addressing both local and global health challenges.
While the advantages of conducting clinical trials in Latin America are compelling, including rich patient diversity and cost-effectiveness, several challenges must be navigated:
- Regulatory hurdles
- Language barriers
- Socioeconomic disparities
These present obstacles that require careful planning and collaboration with local experts. Overcoming these challenges is critical for maximizing the benefits of the region's diverse populations and enhancing the relevance of research findings.
Looking to the future, emerging trends in technology integration, personalized medicine, and international collaboration signal a promising direction for Latin American medical research. Strengthening partnerships among academic institutions, industry stakeholders, and government agencies will be vital in enhancing research capacity and fostering innovation. As the region continues to position itself as a strategic hub for clinical research, the collective efforts to address barriers and leverage opportunities will not only advance medical science but also improve health outcomes for communities across the globe. The evolution of this landscape is not just a regional development; it represents a pivotal moment in the global medical research arena.




