Overview
The advantages of clinical research in Latin America include cost-effectiveness, diverse patient populations, and supportive regulatory frameworks, making the region an attractive site for clinical trials. The article highlights that lower operational costs, a rich mix of ethnic backgrounds for broader research applicability, and expedited approval processes foster a conducive environment for medical studies, thereby enhancing both public health outcomes and economic growth in the region.
Introduction
As the global landscape of clinical research evolves, Latin America emerges as a pivotal region offering unique advantages for conducting clinical trials. With its cost-effective operational environment, diverse patient populations, and progressive regulatory frameworks, the region is becoming increasingly attractive to pharmaceutical companies and research organizations.
The collaboration between local stakeholders and international partners further enhances its appeal, fostering innovation and efficiency in trial processes. However, while opportunities abound, researchers must also navigate the complexities of varying regulations, ethical considerations, and cultural dynamics.
Understanding these factors is crucial for leveraging the benefits of clinical research in Latin America and ensuring successful outcomes that can contribute to global health advancements.
Key Advantages of Clinical Research in Latin America
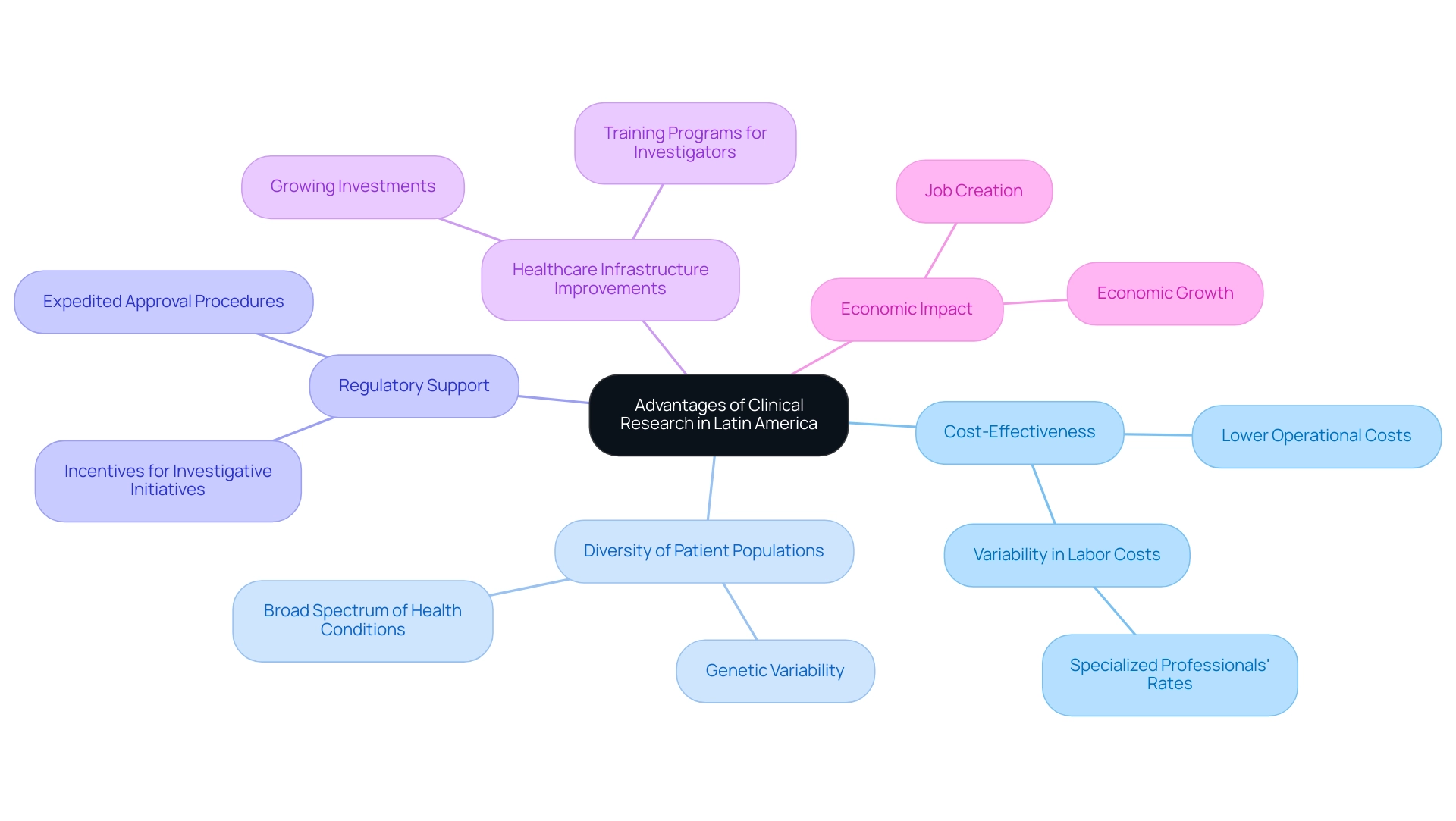
The advantages of clinical research in Latin America offer a variety of important benefits that make it an attractive location for conducting clinical trials. One of the key advantages of clinical research in Latin America is the cost-effectiveness of operations in this region, where expenses are often markedly lower than those in North America and Europe. This reduction in operational costs can result in substantial savings for pharmaceutical companies and research organizations, enabling them to allocate resources more efficiently.
Notably, labor expenses demonstrate considerable variability across nations, especially in the rates for specialized professionals, further enhancing the cost-effectiveness of conducting studies in this region.
Additionally, the diversity of patient populations across Latin America showcases the advantages of clinical research in Latin America. The region is characterized by a rich mix of ethnicities and backgrounds, providing researchers access to a broad spectrum of genetic variations and health conditions. This diversity is vital for developing new treatments that are effective across different demographics, thus enhancing the generalizability of trial results and making findings more applicable to global populations.
The collaboration between bioaccess™ and Caribbean Health Group (CHG) to position Barranquilla as a leading destination for trials was announced on March 29, 2019, during a meeting in Miami, FL. This initiative, backed by Colombia's Minister of Health, seeks to simplify the medical investigation process, promoting an atmosphere favorable to development and creativity. Additionally, nations in Latin America have made considerable advancements in establishing favorable regulatory frameworks for medical studies.
Governments increasingly acknowledge the advantages of clinical research in Latin America, recognizing its significance for public health and economic progress, which has resulted in expedited approval procedures and various incentives for investigative initiatives. This regulatory support not only encourages a more favorable environment for conducting studies but also significantly accelerates timelines for inquiry. Moreover, the region is witnessing growing investments in healthcare infrastructure and study capabilities, which are contributing to the advantages of clinical research in Latin America and further enhancing its appeal.
Many nations are enhancing their medical resources and creating extensive training programs for investigators, which contribute to higher quality results. The collaboration with GlobalCare Clinical Studies illustrates this trend, leading to over a 50% decrease in recruitment duration and a 95% retention rate in studies. As demonstrated by Latin America's 2.1% portion of the worldwide medical studies market in 2023, with expectations for significant expansion, the region is situated as an essential participant in the global medical exploration arena.
Continuous funding and growing global interest further boost the appeal of Latin America for studies, paving the way for better results and wider public health effects. Moreover, the effect of these medical studies on local economies is substantial, with job creation and economic growth serving as direct advantages of the heightened research activities. Comprehensive Clinical Study Management Services, including feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting, are essential components that support this growth and ensure high-quality outcomes.
Challenges and Considerations in Latin American Clinical Trials
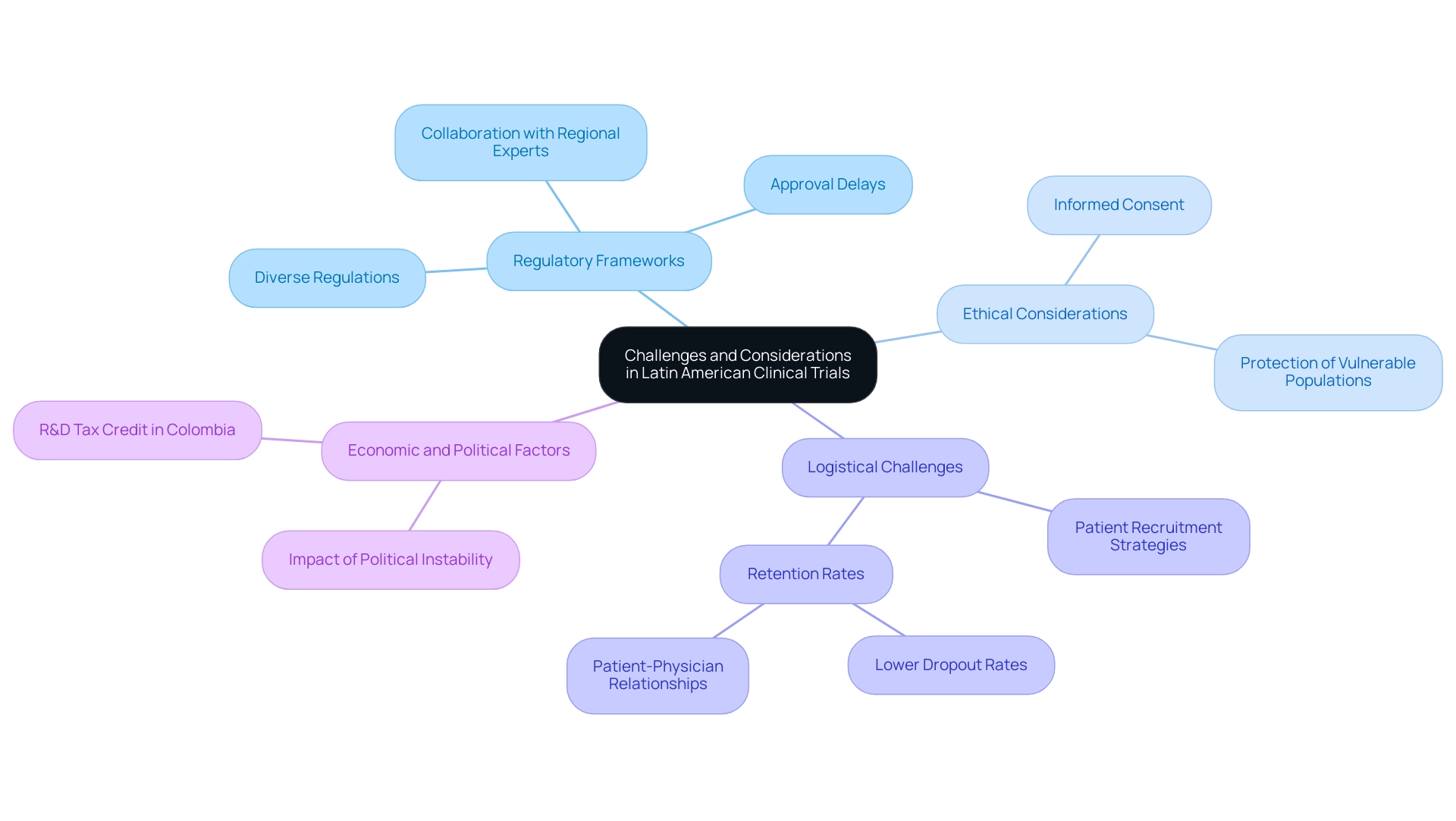
Carrying out medical research in Latin America provides numerous advantages of clinical research in Latin America; however, it is crucial to recognize the related challenges and considerations. A significant hurdle is the variability in regulatory frameworks across the region. Each nation has distinct regulations governing clinical studies, which can complicate the approval process and lead to delays.
Navigating these diverse regulations demands a comprehensive understanding of regional laws and often necessitates collaboration with regional experts. Our service capabilities include:
- Feasibility and selection of research locations
- Thorough reviews and feedback on study documents to ensure adherence to country-specific requirements
- Setup and approval from ethics committees and health ministries
- Management of import permits for investigational devices, which involves the nationalization of devices to comply with regional regulations
Our project management services also cover detailed reporting on study status, including serious and non-serious adverse events, ensuring transparency and compliance throughout the trial process. As noted by Mariana Bei, Sr. Director of Clinical Operations and Brazil GMBA at Parexel,
Having a regional presence and leadership that is based in the LATAM region strengthens the relationships that Parexel builds with regional talent, regulatory authorities based in the area, regional associations and the global network of sites overall.
This regional expertise is invaluable in overcoming regulatory challenges.
Ethical considerations are paramount in clinical research, particularly concerning informed consent and the protection of vulnerable populations. Following ethical standards is essential for preserving the integrity of studies and fostering trust within communities. Researchers must be particularly diligent in these areas to ensure compliance and respect for participants.
Logistical challenges surrounding patient recruitment and retention also warrant attention. Although the diversity of the population can be advantageous, it can complicate the identification of suitable participants who meet specific trial criteria. Effective recruitment strategies that resonate with local populations and address cultural sensitivities are essential for success. Notably, experts agree that the advantages of clinical research in Latin America include lower dropout rates, which are one-third of those in the U.S. and EU, attributed to strong patient-physician relationships and urban concentrations of subjects.
This stability is further supported by our comprehensive project management services and monitoring capabilities, which ensure timely reporting on study status and adverse events.
Lastly, the potential for economic and political instability in certain Latin American countries can impact study timelines and funding availability. However, the new Colombian R&D tax credit enables small and midsize companies to claim a 50% tax credit on their R&D and innovation projects, which showcases the advantages of clinical research in Latin America by providing a financial incentive for undertaking studies in the region. Stakeholders must prepare to adapt to these changing circumstances and devise contingency plans to mitigate risks associated with conducting assessments in these environments.
Overall, although the area presents many possibilities for medical studies, recognizing the advantages of clinical research in Latin America and understanding the related challenges, backed by our extensive management services for studies, is essential for achievement.
Regulatory Frameworks and Compliance in Latin America
In Latin America, regulatory frameworks overseeing research vary significantly across countries, necessitating that researchers possess a thorough understanding of the specific regulations in each jurisdiction. Colombia stands out as an appealing location for first-in-human studies, demonstrating the advantages of clinical research in Latin America, such as cost efficiency—where savings can exceed 30% compared to studies conducted in North America or Western Europe—and a swift regulatory process, with IRB/EC and INVIMA approvals typically completed within 90-120 days. The high-quality healthcare system in Colombia, which ranks 22nd by the World Health Organization among 191 countries, and has hospitals recognized as some of the best in Latin America by 'America Economia,' demonstrates the advantages of clinical research in Latin America, as patient recruitment is facilitated by a population exceeding 50 million, with nearly 95% covered by universal healthcare, ensuring a diverse participant pool.
R&D tax incentives further enhance the appeal of the advantages of clinical research in Latin America, offering substantial financial benefits for investments in science and technology.
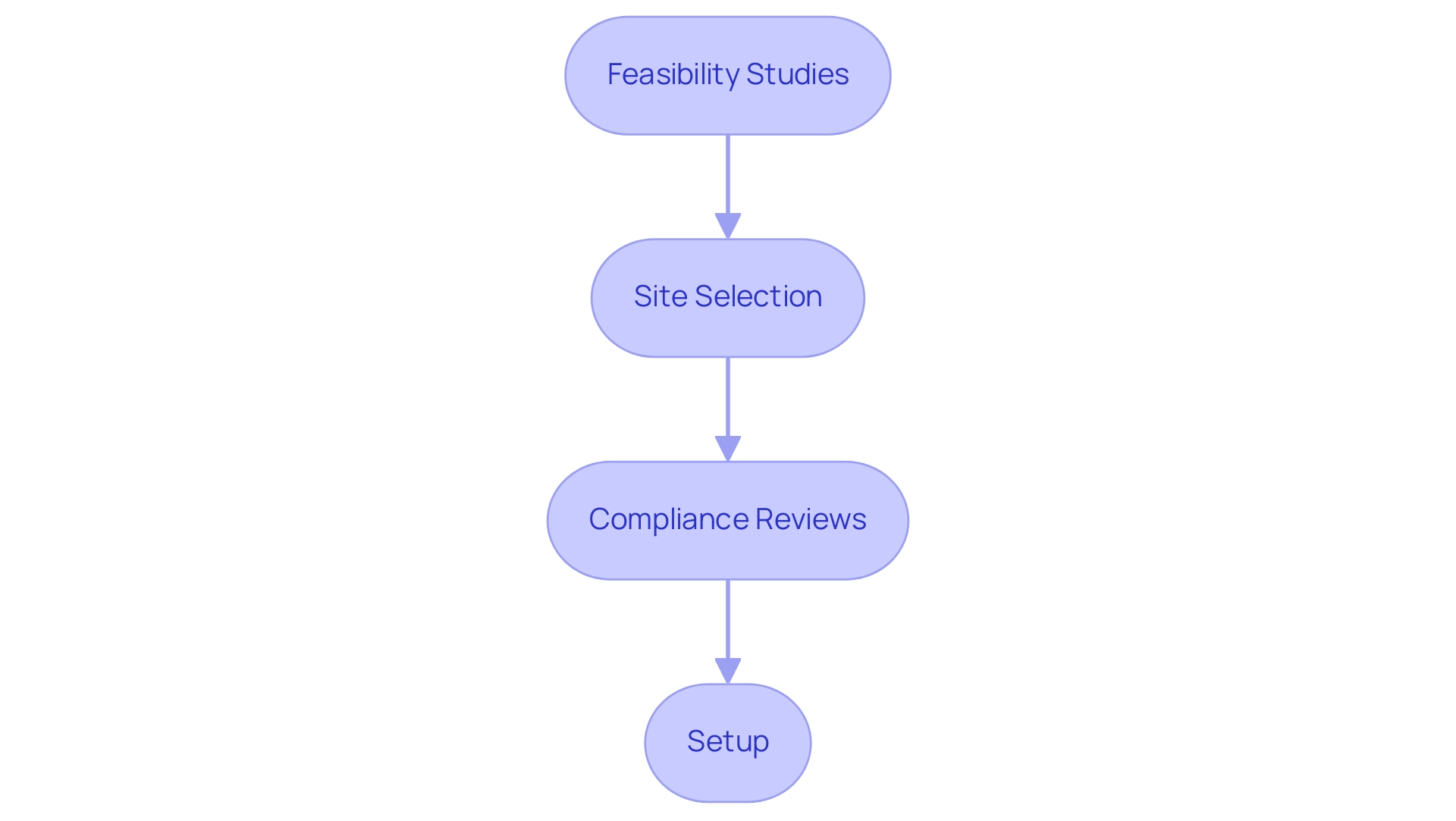
The process for obtaining study approval in Colombia includes comprehensive steps such as:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
All managed by experienced professionals. Regulatory approvals involve a rigorous review by both the ethics committee and health ministry, alongside necessary import permits for investigational devices. This comprehensive method guarantees that studies not only fulfill compliance criteria but are also performed proficiently and effectively.
Our study management services include:
- Feasibility assessments
- Selection of study locations
- Project oversight
Ensuring a smooth process from beginning to end. Katherine Ruiz, an expert in regulatory affairs for medical devices and in vitro diagnostics in Colombia, plays a crucial role in navigating these complexities. Her extensive knowledge and expertise ensure that research studies in Colombia are conducted with the highest standards of integrity and compliance.
As the regulatory landscape evolves, remaining informed about these changes is essential for researchers to ensure compliance and mitigate risks that could compromise their studies.
Cultural Considerations in Conducting Clinical Research
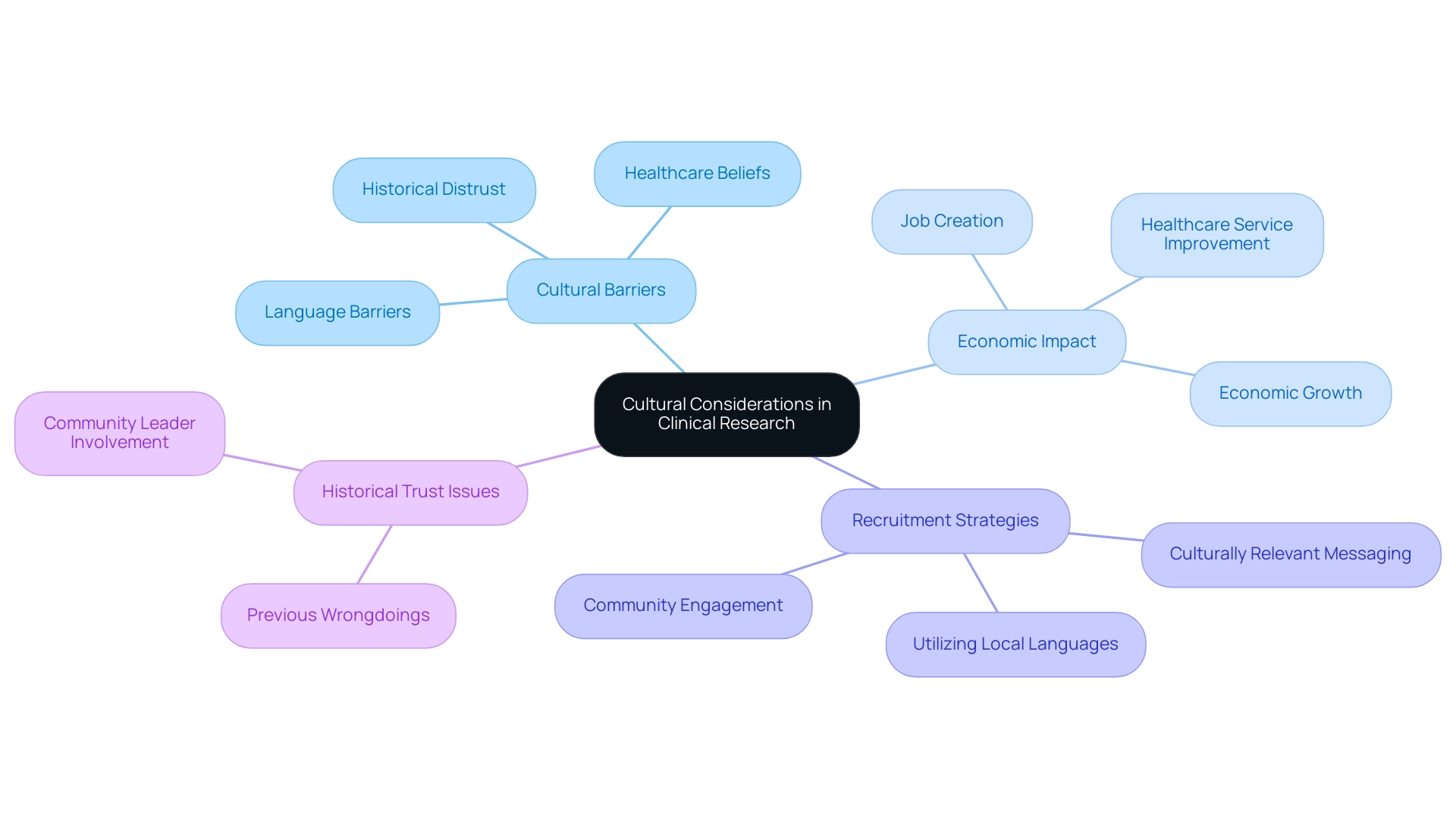
Cultural factors are crucial in carrying out studies in Latin America, as they greatly affect participant involvement and the overall effectiveness of projects. Researchers must be aware of the diverse cultural differences within various communities, including factors such as language barriers, healthcare beliefs, and perspectives on research studies. Dr. Amelie G. Ramirez stresses the significance of tackling these obstacles, pointing out that this study, among the initial ones to recognize South Texas patients’ hurdles to join EPCTs, underscores potential key areas to enhance involvement of both minority and non-minority patients in studies.
This insight is critical, given that even a 1% reduction in health disparities through improved representation could yield over $100 billion in savings related to diabetes and heart disease. Significantly, the involvement of white patients in FDA-approved medications varied from 84 percent in 2014 to 73.7 percent in 2020, highlighting the necessity for improved representation among diverse populations.
In Colombia, initiatives such as the partnership between bioaccess™ and Caribbean Health Group seek to establish Barranquilla as a prominent hub for research in Latin America, emphasizing the advantages of clinical research in Latin America, and are backed by Colombia's Minister of Health. This collaboration is essential in improving the regional scientific environment and promoting international partnerships. Furthermore, media exposure by platforms such as Clinical Leader has emphasized the increasing significance of medical studies in the area, presenting success narratives and the possible advantages of these initiatives.
Nonetheless, a historical distrust of medical investigations exists in specific regions, arising from previous wrongdoings, which requires a clear and respectful method to establish confidence with prospective participants. Involving community leaders and stakeholders is essential for closing these gaps and promoting a more positive view of research initiatives. For instance, a case study on NIH-supported trials examined racial and ethnic participation, revealing variability in participation rates among different demographics, which highlights the importance of cultural considerations in recruitment strategies.
Furthermore, the advantages of clinical research in Latin America highlight the economic impact of clinical trials, which cannot be overlooked, as these studies are known to create jobs, stimulate economic growth, and enhance healthcare services within communities. Researchers should adapt their recruitment strategies to align with regional customs and communication styles. This may involve utilizing local languages, crafting culturally relevant messaging, and being sensitive to the values and norms of the communities involved.
It is also important to acknowledge the limitations of current studies, including non-representative samples and the need for further investigation to understand the experiences of those who declined participation in Pets. By prioritizing cultural competence, researchers can enhance participant recruitment and retention, which ultimately leads to more robust and reliable study outcomes.
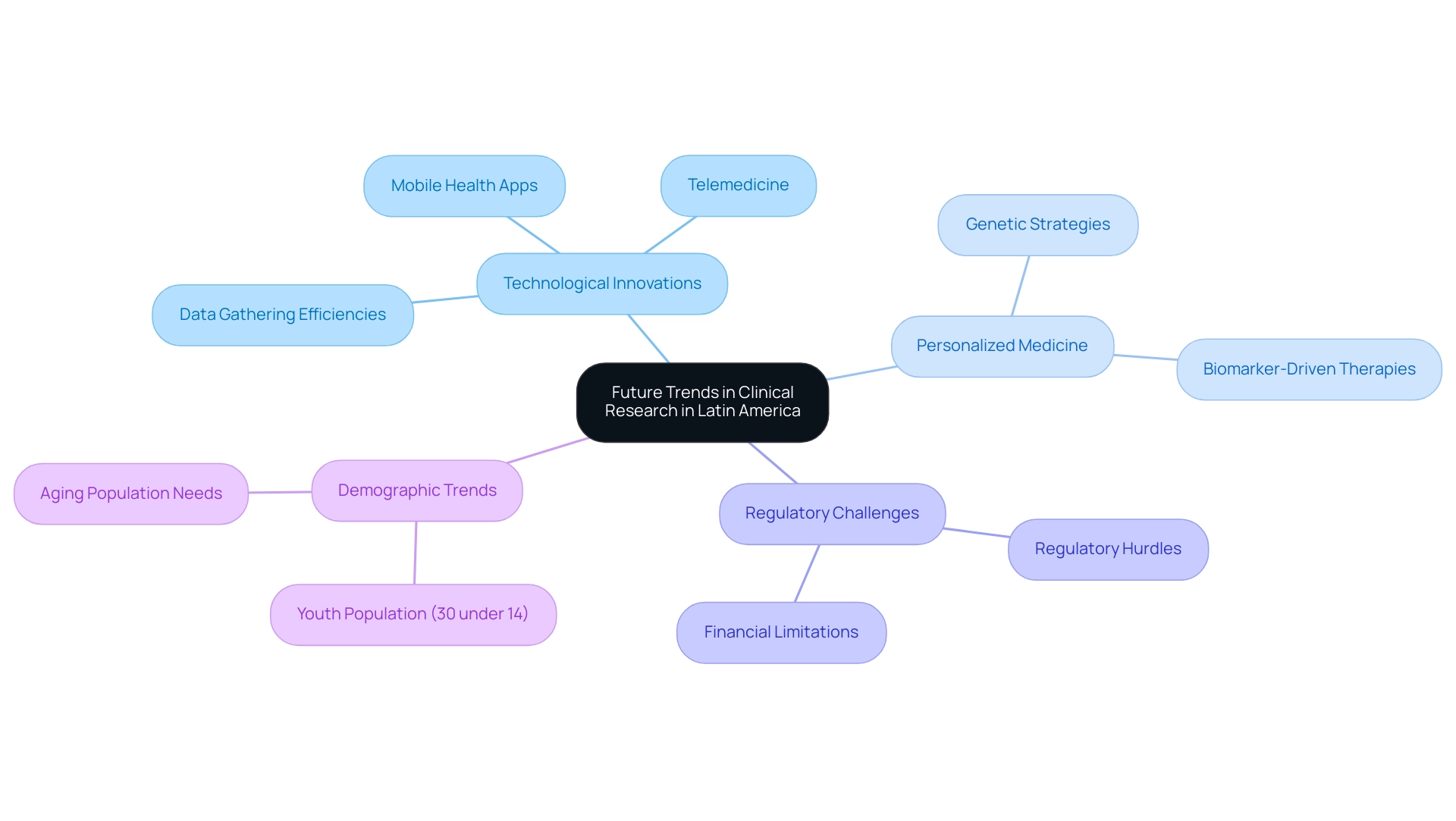
Future Trends in Clinical Research in Latin America
The future of medical research in Latin America is poised to experience transformative advancements, driven by a wave of technological innovations and a growing focus on personalized medicine, highlighting the advantages of clinical research in Latin America. Digital health technologies, including telemedicine and mobile health applications, are transforming the environment of research studies, enabling more efficient patient recruitment and data gathering. These tools not only improve accessibility but also simplify processes, making tests more efficient.
However, Medtech companies in the region face significant challenges, including regulatory hurdles, limited financial resources, and prolonged subject recruitment timelines. These problems can impede the advancement of research studies and necessitate creative solutions to address. Moreover, the shift towards personalized medicine is prompting researchers to delve into genetic and biomarker-driven strategies, paving the way for targeted therapies that promise improved patient outcomes.
This focus aligns with the region's demographic trends, where approximately 30% of the population is under 14 years old, highlighting the future shift towards an aging population that will require tailored medical solutions, indicating a growing market for new drug developments aimed at older patients.
Collaborative initiatives among research institutions, pharmaceutical companies, and regulatory bodies are anticipated to cultivate a more integrated research ecosystem, which showcases the advantages of clinical research in Latin America. Notably, partnerships like that between Greenlight Guru and bioaccess™ aim to accelerate Medtech innovations and clinical trials in the region. As emphasized by Mariana Bei, Sr. Director of Clinical Operations and Brazil GMBA at Parexel, "Having a regional presence and leadership that is situated in the LATAM area enhances the connections that Parexel establishes with regional talent and regulatory authorities."
This local expertise is essential for navigating the evolving regulatory environments, as demonstrated by the success of local regulatory services in expediting approval timelines and enabling simultaneous market launches, such as seen in the case of Parexel's collaborations with local CROs and associations like ABRACRO.
The comprehensive service capabilities available encompass feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting, which are essential for tackling the challenges encountered by Medtech companies. As Latin America strengthens its regulatory systems, including the supervision by INVIMA, the Colombia National Food and Drug Surveillance Institute, and invests in development infrastructure, the advantages of clinical research in Latin America will position it to attract a growing number of international trials. This influx will not only enrich the local healthcare landscape but also contribute significantly to global medical advancements, affirming Latin America's vital role in the clinical research domain while also generating economic benefits through job creation and healthcare improvement.
Conclusion
The clinical research landscape in Latin America presents a dynamic and promising environment for pharmaceutical companies and research organizations. With its cost-effective operational framework, diverse patient populations, and progressive regulatory support, the region is increasingly recognized as an attractive destination for conducting clinical trials. The collaboration between local stakeholders and international partners further enhances this appeal, fostering innovation and improving research efficiency.
However, the advantages of conducting clinical trials in Latin America are accompanied by challenges that must be navigated carefully. Variability in regulatory frameworks, ethical considerations, and logistical hurdles can complicate trial processes. A thorough understanding of local regulations and cultural dynamics is essential for ensuring compliance and fostering trust among participants. By addressing these challenges with effective strategies, researchers can maximize the benefits of the region's unique attributes.
Looking ahead, the future of clinical research in Latin America is poised for transformative growth, driven by technological innovations and an emphasis on personalized medicine. As the region continues to strengthen its regulatory frameworks and invest in healthcare infrastructure, it is well-positioned to attract more international clinical trials. This evolution not only promises to enhance the quality of research outcomes but also to foster economic growth and improvements in public health.
In summary, Latin America stands as a pivotal player in the global clinical research arena. By leveraging its advantages and addressing the associated challenges, stakeholders can contribute to significant advancements in healthcare, ensuring that the region remains a vital contributor to global medical progress.
Frequently Asked Questions
What are the main advantages of conducting clinical research in Latin America?
The main advantages include cost-effectiveness, diverse patient populations, favorable regulatory frameworks, growing healthcare infrastructure, and significant economic impacts such as job creation.
How does cost-effectiveness play a role in clinical research in Latin America?
Operational costs in Latin America are often lower than in North America and Europe, allowing pharmaceutical companies and research organizations to save money and allocate resources more efficiently.
Why is the diversity of patient populations in Latin America beneficial for clinical trials?
The region's diverse mix of ethnicities and backgrounds provides access to various genetic variations and health conditions, enhancing the generalizability of trial results and making findings more applicable to global populations.
What initiatives have been taken to promote clinical trials in Latin America?
A collaboration between bioaccess™ and Caribbean Health Group (CHG) aims to position Barranquilla as a leading destination for trials, supported by Colombia's Minister of Health to simplify the medical investigation process.
How are regulatory frameworks in Latin America evolving to support clinical research?
Governments are recognizing the significance of clinical research for public health and economic progress, leading to expedited approval procedures and various incentives for research initiatives.
What impact does clinical research have on local economies in Latin America?
Clinical research activities contribute to job creation and economic growth, providing direct benefits to local communities.
What challenges are associated with conducting clinical research in Latin America?
Challenges include variability in regulatory frameworks, ethical considerations, logistical issues in patient recruitment and retention, and potential economic and political instability.
How does the variability in regulatory frameworks affect clinical trials in Latin America?
Each nation has distinct regulations, complicating the approval process and potentially leading to delays, which requires a comprehensive understanding of regional laws.
What ethical considerations must researchers keep in mind during clinical trials?
Researchers must ensure informed consent and protect vulnerable populations, adhering to ethical standards to maintain the integrity of studies and build trust within communities.
How do patient recruitment and retention differ in Latin America compared to other regions?
Latin America has lower dropout rates than the U.S. and EU, attributed to strong patient-physician relationships, although cultural sensitivities must be addressed for effective recruitment strategies.
What financial incentives are available for conducting research in Latin America?
The new Colombian R&D tax credit allows small and midsize companies to claim a 50% tax credit on their R&D and innovation projects, encouraging studies in the region.

