Overview
The primary compliance risks in trial design encompass:
- Informed consent issues
- Data integrity concerns
- Protocol deviations
- Deficiencies in staff training
Addressing these risks is essential for upholding ethical standards and ensuring the validity of research outcomes. Poor adherence can result in significant safety concerns, regulatory scrutiny, and compromised study integrity. It is imperative for stakeholders in the clinical research landscape to recognize these challenges and take proactive measures to mitigate them.
Introduction
In the intricate world of clinical trials, compliance transcends a mere regulatory checkbox; it stands as the cornerstone of ethical research and participant safety. As the landscape of clinical research evolves, so too do the challenges associated with adhering to Good Clinical Practice (GCP) guidelines and regulatory requirements. From informed consent issues to data integrity concerns and protocol deviations, the risks are multifaceted and can profoundly impact trial outcomes.
This article delves into the critical compliance risks that researchers must navigate, emphasizing best practices and effective strategies for risk management. By prioritizing compliance and fostering a culture of accountability, clinical researchers can enhance trial integrity and contribute to the advancement of medical technologies, ultimately improving healthcare outcomes.
Understanding Compliance Risks in Clinical Trial Design
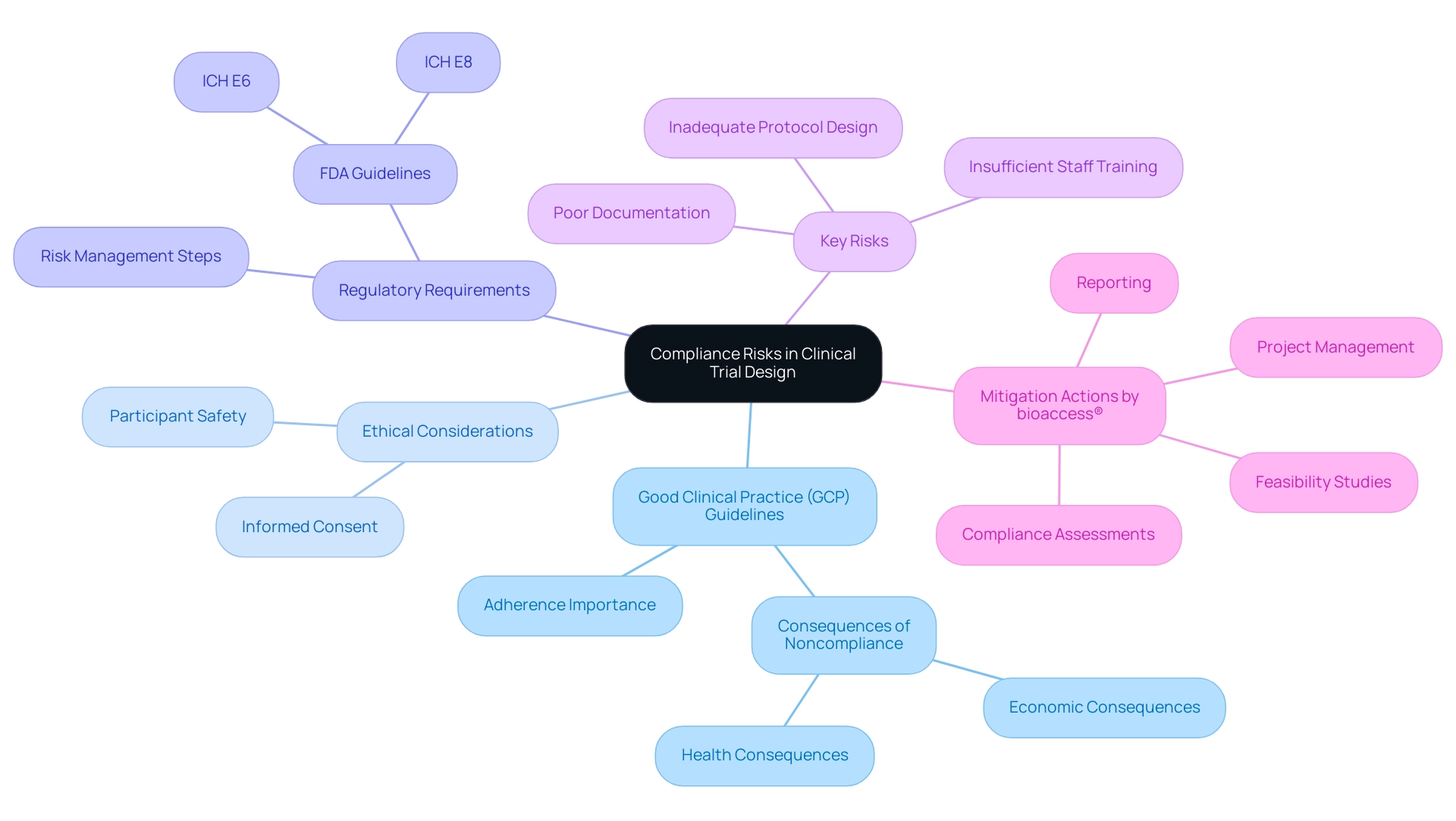
Compliance risks in trial design encompass a variety of critical issues, including adherence to Good Clinical Practice (GCP) guidelines, ethical considerations, and regulatory requirements. These risks can stem from factors such as inadequate protocol design, insufficient staff training, or failure to maintain accurate documentation. Recognizing and understanding these risks is essential for effective mitigation, ensuring that experiments are conducted ethically and efficiently.
With over 20 years of experience in the Medtech sector, bioaccess® underscores the significance of strict adherence to improve study integrity and results through its extensive research management services. These services include:
- Feasibility studies
- Site selection
- Compliance assessments
- Setup
- Import permits
- Project management
- Reporting
For instance, a poorly designed protocol can lead to significant participant safety concerns, which may trigger regulatory scrutiny and result in delays. Recent statistics indicate that noncompliance can have severe economic and health consequences, underscoring the importance of rigorous adherence to GCP standards. Phil Skolnick, a notable figure in medical research, emphasizes that "noncompliance is also pertinent in evaluating adverse events in research studies, before the development cycle of a medication might lead to potentially disastrous economic and health outcomes."
Moreover, the FDA's guidelines, particularly ICH E6 and ICH E8, have established comprehensive steps for integrating risk management into clinical studies since the late 1980s. This framework assists researchers in recognizing adherence risks early in the study design process, thereby protecting the integrity of their investigations.
A practical alternative for evaluating conformity is measuring drug presence at regular intervals, which can provide valuable insights into adherence and participant engagement. Furthermore, a systematic review titled "Statistical Methods for Noncompliance in RCTs" highlights various causal inference techniques, such as Rubin's causal model and instrumental variables, developed to address noncompliance in randomized studies involving medical devices. This review elucidates the degree to which these statistical techniques are utilized in device intervention studies, contributing to more precise estimates of treatment effects and ultimately assisting in the reduction of adherence risks.
In summary, understanding compliance risks in trial design is essential. By prioritizing GCP adherence and employing robust statistical methods, researchers can enhance research outcomes and ensure the ethical conduct of their investigations. Moreover, the influence of Medtech research extends beyond the studies themselves, promoting job creation, economic development, healthcare enhancement, and international cooperation within local economies.
Identifying Key Compliance Risks in Clinical Trials
Key compliance risks in clinical trials encompass several critical areas that require vigilant attention:
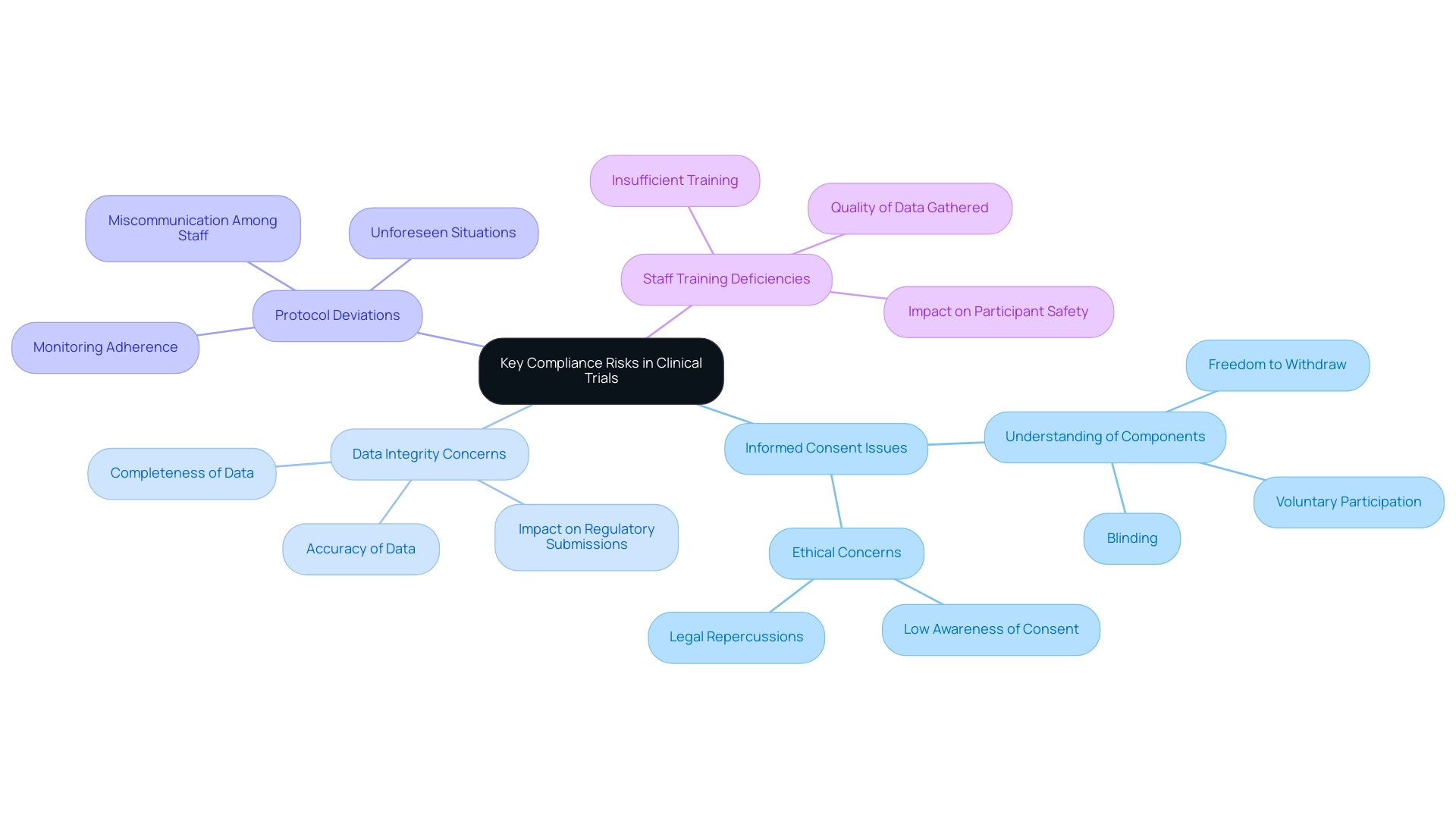
- Informed Consent Issues: The failure to secure proper informed consent can lead to significant ethical violations and legal repercussions. Recent findings indicate that over 50% of research participants struggle to fully understand key components of informed consent, such as voluntary participation, blinding, and the freedom to withdraw. K.S. noted that the level of comprehension regarding these components was low, being understood by only half of the patients. This lack of understanding raises serious concerns about the ethical integrity of studies, especially considering that most research participants showed low awareness of what they consented to.
- Data Integrity Concerns: The accuracy and completeness of data are paramount in medical research. Inaccurate or incomplete data can severely compromise trial results and regulatory submissions, leading to potential setbacks in the approval process for medical devices. Recent conversations among specialists emphasize that preserving data integrity is vital for guaranteeing the reliability of research findings. With over 20 years of experience in the Medtech sector, bioaccess® highlights the significance of strong data management practices, especially in the context of accelerated medical device clinical trial services in Latin America.
- Protocol Deviations: Deviations from the approved study protocol can jeopardize compliance and the validity of study outcomes. Such deviations may arise from various factors, including miscommunication among staff or unforeseen situations during the experiment. It is essential for researchers in the medical field to monitor adherence to protocols closely to mitigate these risks, a practice that bioaccess® integrates into its comprehensive management services, which include Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF).
- Staff Training Deficiencies: Insufficient training of clinical study personnel can result in mistakes in execution, which may affect participant safety and the quality of data gathered. Ensuring that all personnel are well-trained and informed about trial protocols and regulatory requirements is crucial for reducing risks. bioaccess® is dedicated to offering comprehensive training and support to ensure that all team members are prepared to maintain the highest standards.
The case study titled "Impact of Research Quality on COVID-19 Publications" examined the relationship between informed consent reporting and the quality of research, noting that the rapid increase in COVID-19 publications may have compromised study quality due to the urgency of obtaining results. This underscores the need for a balance between speed and thoroughness in research practices.
By systematically identifying compliance risks in trial design, researchers can implement effective strategies to mitigate them, thereby enhancing study outcomes and ensuring adherence to ethical standards. bioaccess®'s commitment to data protection, transparent grievance procedures, and addressing client concerns further reinforces client trust in navigating these challenges.
Navigating Regulatory Requirements and Their Impact on Compliance
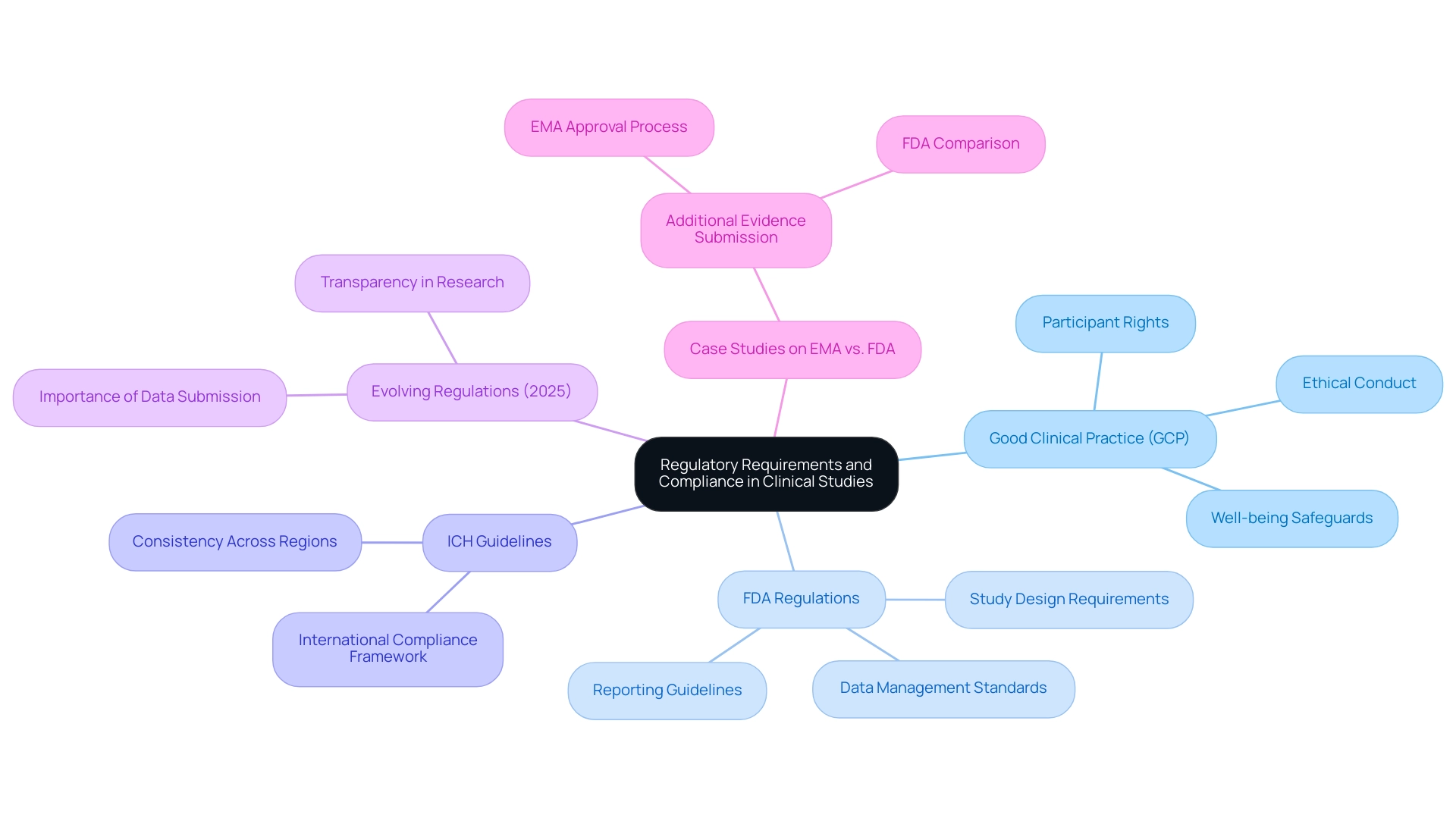
Regulatory requirements for clinical studies are established by various authorities, including the FDA and EMA, and are crucial in shaping how studies are designed, conducted, and reported. Key regulations include:
- Good Clinical Practice (GCP): This set of guidelines ensures that trials are conducted ethically, safeguarding participant rights and well-being throughout the research process.
- FDA Regulations: These regulations outline specific requirements for study design, data management, and reporting, ensuring that studies meet rigorous standards for safety and efficacy.
- ICH Guidelines: The International Council for Harmonization (ICH) offers a framework for international compliance, facilitating the execution of global studies and promoting consistency across various regulatory environments.
Staying informed about compliance risks in trial design is crucial for researchers, as failing to comply can lead to severe consequences, including termination of the study and potential legal repercussions. Statistics show that a substantial percentage of research studies are halted due to compliance risks in trial design, emphasizing the vital necessity for adherence to established guidelines.
In 2025, regulatory requirements continue to evolve, with both the FDA and EMA emphasizing the importance of robust data submission and transparency in clinical research processes. Recent insights from professionals in the field emphasize that understanding these evolving regulations is essential for successful study design. For instance, Roberta Joppi from the Clinical Research and Drug Assessment Unit emphasizes, "Later availability of new therapeutics may disappoint needy patients and their physicians," underscoring the urgency of compliance in the approval process.
Furthermore, the EMA's recent publication on February 10, 2023, highlights the necessity for further evidence in the approval process, which can significantly influence study outcomes.
Case studies, such as the examination of additional evidence submissions to the EMA compared to the FDA, reveal that only a minority of medicines had supplementary information by the time of EMA approval. This indicates that although the EMA's approval process gains from extra data, differences in efficacy conclusions between the two agencies can result in varied regulatory outcomes, highlighting compliance risks in trial design.
By staying updated on regulatory changes and applying Good Clinical Practice effectively, researchers can modify their study designs to meet adherence requirements, ultimately promoting medical devices and therapeutics more efficiently. The knowledge and tailored method of bioaccess® play a crucial role in assisting Medtech companies maneuver through these complexities, ensuring that trials are conducted in accordance with current regulatory standards. Our extensive research project management services include feasibility studies, site selection, regulatory reviews, study setup, import permits, project management, reporting (covering study status, inventory, and serious and non-serious adverse events), and review and feedback on study documents to meet country requirements, all designed to assist our clients in attaining regulatory excellence.
Furthermore, bioaccess® is committed to ensuring information security and client trust, addressing any concerns through our grievance and data protection procedures.
Common Challenges in Ensuring Compliance During Trials
Ensuring adherence during clinical studies presents several typical compliance risks in trial design that can significantly affect the integrity and results of research. Key issues include:
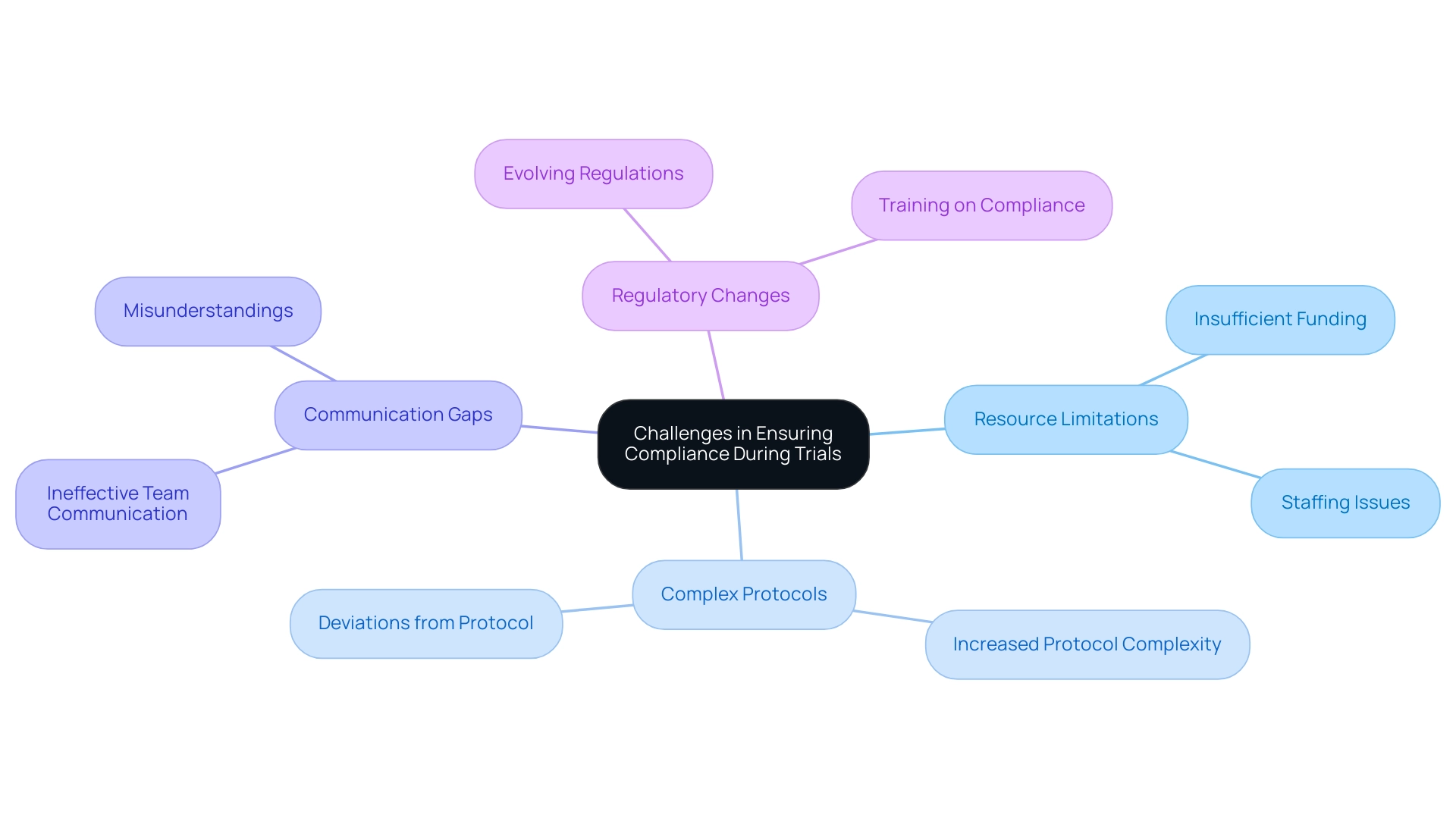
- Resource Limitations: Insufficient funding and staffing are prevalent obstacles that can severely hinder compliance efforts. These limitations often lead to oversight and errors, compromising validity.
A notable statistic reveals that 71% of studies showed no change in significance in results between intention-to-treat (ITT) analysis and compliance-handling analysis, underscoring the critical nature of resource allocation in maintaining compliance.
- Complex Protocols: As research studies evolve, protocols become increasingly intricate. This complexity can create compliance risks in trial design, making it difficult for research teams to adhere to every aspect of the protocol and increasing the likelihood of deviations that could affect trial outcomes.
- Communication Gaps: Effective communication is vital in trials. Ineffective communication among team members can result in misunderstandings and protocol deviations, jeopardizing adherence. Establishing clear communication channels is essential for mitigating these risks.
- Regulatory Changes: The landscape of clinical research continually shifts due to evolving regulations. Keeping abreast of compliance risks in trial design can be particularly daunting for smaller organizations, which may lack the resources to adapt swiftly. Regular training and updates on regulatory requirements are crucial for minimizing compliance risks in trial design. This insight is especially pertinent for site selection and regulatory considerations, as it underscores the significance of qualified personnel in upholding research integrity.
Tackling these challenges requires a proactive approach. Implementing regular training sessions, fostering open communication, and employing robust project management practices can significantly enhance adherence efforts. Bioaccess provides extensive management services for studies, including feasibility assessments, site selection, regulatory reviews, setup, import permits, project management, and reporting on serious and non-serious adverse events, which are crucial in navigating these complexities.
Additionally, sensitivity analyses should be conducted to assess the impact of missing data assumptions, particularly in scenarios where data may be missing not at random (MNAR). As noted by the FDA, for studies affected by the pandemic, evaluating the shift in the benefit/risk for participants is the first step in the decision-making process. This thorough approach not only assists in managing the intricacies of regulations but also guarantees the integrity and validity of research studies.
Moreover, the findings from the case analysis on COVID-19's influence on medical experiments emphasize the need for continuous risk evaluations and record-keeping to uphold initial project goals while adjusting to interruptions. The effect of Medtech research goes beyond regulations, aiding local economies through job creation, economic growth, and healthcare enhancement, promoting international cooperation in the process.
Implementing Effective Risk Management Strategies
Implementing effective risk management approaches in clinical studies is vital for guaranteeing adherence and improving study quality. This process encompasses several key steps that align with the seven key steps of the Risk-Based Quality Management (RBQM) process:
- Risk Assessment: Regularly evaluating potential compliance risks throughout the trial lifecycle is crucial. This proactive approach allows researchers to identify vulnerabilities early, enabling timely interventions that can mitigate risks before they escalate.
- Training and Education: Ongoing training for staff is essential to ensure that all team members are well-versed in regulatory requirements and best practices. An informed group is better prepared to manage the intricacies of clinical studies, thereby decreasing the chances of regulatory problems.
- Monitoring and Auditing: Conducting regular audits is a fundamental practice for identifying compliance issues at an early stage. These audits not only help in addressing problems promptly but also foster a culture of accountability and transparency within the research team. Research has indicated that regular oversight can result in a substantial decrease in compliance-related mistakes, improving the overall integrity of the study.
- Documentation: Maintaining comprehensive documentation of all activities is essential for ensuring transparency and accountability. Detailed records offer a clear path of adherence efforts and can be invaluable during audits or regulatory reviews.
Alongside these strategies, bioaccess provides extensive management services for studies, encompassing feasibility assessments, site selection, regulatory reviews, setup, import permits, project oversight, and reporting. These services are designed to assist researchers in managing the intricacies of study design and implementation, ultimately improving adherence and minimizing risks.
Clinical studies can be categorized into several types, including prevention studies, diagnostic studies, screening studies, treatment studies, and quality of life studies, each serving different purposes in health and medicine. By implementing these strategies, clinical researchers can significantly lower adherence risks, thereby addressing compliance risks in trial design and enhancing the overall quality of their studies. For instance, a recent case study titled 'Adapting Clinical Studies to Modern Challenges' highlighted how the integration of centralized monitoring and real-time data analytics improved oversight, reduced the need for on-site visits, and provided sponsors with quicker insights into study progress.
This shift not only streamlined operations but also strengthened adherence through enhanced data accuracy and oversight.
As Katherine Ruiz, an expert in regulatory affairs for medical devices and in vitro diagnostics in Colombia, emphasizes, "By submitting, you agree to the processing of your personal data by eClinical Solutions as described in our Privacy Policy." This underscores the significance of adherence and risk management in research studies. In 2025, effective risk management strategies will continue to evolve, integrating advanced technologies and methodologies to tackle the growing complexity of research studies.
As the environment of medical research evolves, staying updated on the newest risk evaluation methods and regulatory requirements will be crucial for success.
The Importance of Training for Compliance Awareness
Training for compliance awareness must encompass several key components to ensure that research personnel are well-equipped to navigate the complexities of study design.
-
Regulatory Updates: Regular updates on changes in regulations and guidelines are essential. Keeping personnel informed about the latest advancements is crucial for addressing compliance risks in trial design. This vigilance ensures adherence to changing standards, which is vital for preserving the integrity of research studies. This is particularly significant given the oversight functions of INVIMA, Colombia's National Food and Drug Surveillance Institute, which plays a pivotal role in regulating trials and medical devices.
-
GCP Training: Comprehensive training on Good Clinical Practice (GCP) is fundamental for ensuring ethical conduct in trials. GCP training not only enhances adherence rates but also cultivates a culture of accountability among clinical research professionals. Research indicates that organizations with robust GCP training programs experience significantly higher adherence rates, underscoring the importance of this training.
-
Role-Specific Training: Tailored training programs designed for specific roles within the research team are critical. By addressing the unique regulatory responsibilities associated with each position, organizations can ensure that all team members comprehend their obligations, thereby minimizing compliance risks in trial design related to non-adherence. This targeted approach has been shown to enhance engagement and retention of essential regulatory information.
-
Scenario-Based Learning: Incorporating real-world situations into training helps staff grasp the practical implications of non-adherence. This method not only illustrates the significance of following protocols but also equips personnel to manage potential adherence challenges effectively.
Investing in these training elements fosters an informed workforce that prioritizes adherence to compliance risks in trial design, ultimately leading to more successful research studies. Recent research emphasizes that organizations prioritizing training for regulatory awareness observe a significant enhancement in trial results, with 73.5% of participants indicating satisfaction with the medical care provided. This statistic reflects the positive impact that well-trained staff can have on the overall success of research initiatives.
However, challenges persist, as highlighted by the case analysis on 'Challenges in Clinical Research Training Adherence,' which underscores the extensive training requirements and the low adherence rates among clinical research professionals. This suggests that current training programs may not fully meet staff needs, emphasizing the necessity for future research to explore more effective engagement strategies. Furthermore, it is crucial to acknowledge the constraints of research in this area, such as the inability to directly evaluate the impact of participation on perceptions and the potential for memory bias in responses.
As Victoria Gonzalez-Nava noted, the initial conceptual framework for research and the overall execution of projects are vital for ensuring adherence and successful outcomes. At bioaccess, we provide extensive services such as feasibility studies, site selection, and regulatory reviews to support these training initiatives, ensuring that research personnel not only adhere to regulations but are also equipped to manage the complexities of study design effectively.
Best Practices for Maintaining Compliance in Clinical Trials
Upholding regulations in clinical studies is essential for guaranteeing the integrity of research results and protecting participant welfare. Key best practices to uphold compliance include:
-
Developing Clear Protocols: Establishing well-defined and accessible trial protocols is essential. Clear protocols guide all team members, ensuring everyone understands their roles and responsibilities, thereby minimizing the risk of non-compliance.
-
Regular Training and Refresher Courses: Continuous education is vital. Implementing ongoing training programs helps keep staff informed about evolving regulations and reinforces the importance of adherence to protocols. As one participant remarked, "this course ought to be accessible to anyone utilizing/collecting data instead of only statisticians," emphasizing the necessity for widespread availability in training.
-
Effective Communication: Open lines of communication among team members are critical. Encouraging discussions about regulatory issues allows for prompt identification and resolution of potential problems, fostering a culture of accountability.
-
Utilizing Technology: The integration of technology in data management and monitoring significantly improves adherence tracking. Advanced tools can automate regulatory checks and provide real-time insights, allowing for swift corrective actions when necessary.
Indeed, a recent examination disclosed that merely 14 out of 158 randomized controlled studies (9%) incorporated adherence as a factor in their assessment, underscoring the importance of adherence in medical studies. By following these optimal methods, medical researchers can create a strong adherence framework that not only aids in the successful implementation of studies but also reduces compliance risks in trial design associated with regulatory violations. For example, bioaccess® provides extensive clinical research management services, including feasibility assessments, site selection, adherence reviews, setup, import permits, project management, and reporting, which are crucial for ensuring regulatory adherence.
Moreover, the case analysis entitled "Impact of Adherence on Treatment Effect Estimates" demonstrated the essential role of adherence in estimating treatment effects in device studies. It emphasized that non-compliance can lead to biased results and advocated for the integration of compliance thresholds into statistical analysis plans. Furthermore, compliance risks in trial design due to non-compliance with regulatory requirements can result in severe legal consequences, including fines and loss of approval.
This proactive approach not only safeguards trial integrity but also aligns with industry standards, ultimately benefiting the advancement of medical technologies.
bioaccess® is committed to ensuring information security and client trust through robust data protection measures and grievance procedures. If clients have any concerns regarding data processing, they can reach out to our Grievance Officer at IMH ASSETS CORP (doing business as "bioaccess®"), ensuring that client concerns are addressed with compliance and transparency.
Conclusion
Navigating the complexities of compliance in clinical trials is essential for safeguarding participant welfare and ensuring the integrity of research outcomes. This article highlights critical compliance risks, including issues related to:
- Informed consent
- Data integrity
- Protocol deviations
- Staff training deficiencies
Each of these areas poses significant challenges that can compromise the ethical conduct of trials and impact overall results.
Effective risk management strategies, such as:
- Regular training
- Thorough documentation
- Ongoing monitoring
are vital for mitigating these risks. By implementing robust compliance frameworks and fostering a culture of accountability, clinical researchers can enhance trial outcomes and uphold the highest ethical standards. The integration of technology and real-time data analytics further strengthens compliance efforts, allowing for timely interventions and improved oversight.
Ultimately, prioritizing compliance not only benefits individual trials but also contributes to the broader advancement of medical technologies and healthcare improvements. As the regulatory landscape continues to evolve, staying informed and adapting to new guidelines will be crucial for researchers. By embracing best practices and leveraging comprehensive clinical trial management services, organizations can navigate these complexities with confidence, ensuring that their studies are conducted ethically and effectively for the benefit of all stakeholders involved.
Frequently Asked Questions
What are compliance risks in trial design?
Compliance risks in trial design include adherence to Good Clinical Practice (GCP) guidelines, ethical considerations, and regulatory requirements, which can arise from inadequate protocol design, insufficient staff training, or failure to maintain accurate documentation.
Why is it important to recognize and understand compliance risks?
Recognizing and understanding compliance risks is essential for effective mitigation, ensuring that experiments are conducted ethically and efficiently, which ultimately improves study integrity and results.
What services does bioaccess® provide to mitigate compliance risks?
bioaccess® offers a range of research management services, including feasibility studies, site selection, compliance assessments, setup, import permits, project management, and reporting.
What are the consequences of poorly designed protocols in clinical trials?
Poorly designed protocols can lead to significant participant safety concerns, regulatory scrutiny, and delays in the trial process.
What are the economic and health consequences of noncompliance in clinical trials?
Noncompliance can have severe economic and health consequences, affecting the approval process for medical devices and potentially leading to disastrous outcomes in research studies.
What guidelines have the FDA established for risk management in clinical studies?
The FDA's guidelines, particularly ICH E6 and ICH E8, provide comprehensive steps for integrating risk management into clinical studies, helping researchers identify adherence risks early in the study design process.
How can measuring drug presence help in evaluating compliance?
Measuring drug presence at regular intervals can provide valuable insights into adherence and participant engagement, aiding in the evaluation of compliance.
What are some key compliance risks identified in clinical trials?
Key compliance risks include informed consent issues, data integrity concerns, protocol deviations, and staff training deficiencies.
What are the implications of informed consent issues in clinical trials?
Informed consent issues can lead to ethical violations and legal repercussions, as many research participants struggle to fully understand key components of informed consent.
Why is data integrity important in medical research?
Data integrity is crucial because inaccurate or incomplete data can compromise trial results and regulatory submissions, potentially delaying the approval process for medical devices.
How do protocol deviations affect clinical trials?
Protocol deviations can jeopardize compliance and the validity of study outcomes, arising from miscommunication or unforeseen situations during the experiment.
What role does staff training play in mitigating compliance risks?
Insufficient training of clinical study personnel can lead to execution mistakes, affecting participant safety and data quality, making comprehensive training essential for reducing risks.
What does bioaccess® do to ensure high training standards for clinical study personnel?
bioaccess® is dedicated to offering comprehensive training and support to ensure that all team members are prepared to maintain the highest standards in clinical trials.
How does the rapid increase in COVID-19 publications relate to research quality?
The urgency to obtain results during the COVID-19 pandemic may have compromised study quality, highlighting the need for a balance between speed and thoroughness in research practices.




