Introduction
In the complex landscape of medical device regulation, the 510(k) cover letter serves as a critical component that can significantly influence the outcome of a submission. This document not only introduces the device to the FDA but also encapsulates essential information that facilitates a smoother review process. With the stakes high and the potential for common pitfalls looming, understanding the key elements of an effective cover letter becomes paramount.
From ensuring clarity and organization to adhering to regulatory guidelines, manufacturers must navigate a myriad of requirements to bolster their chances of approval. This article delves into the fundamental aspects of crafting a compelling 510(k) cover letter, highlighting best practices and common mistakes to avoid, ultimately paving the way for successful submissions in the ever-evolving medical device industry.
Key Components of a 510(k) Cover Letter
A well-crafted 510(k) cover letter is essential for facilitating the FDA's review process, and it must contain several key components:
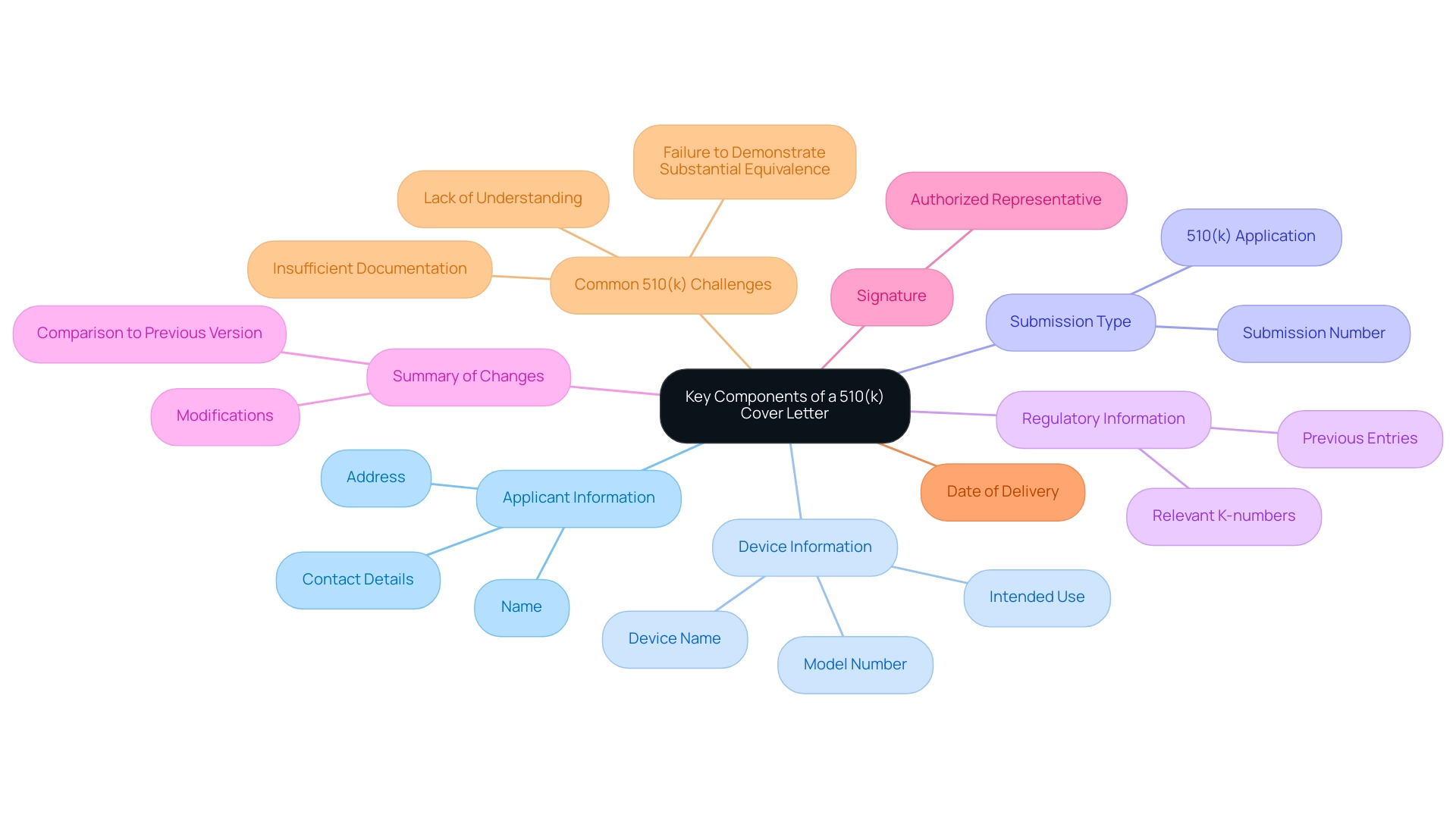
- Applicant Information: Clearly provide the name, address, and contact details of the submitting applicant or organization.
- Device Information: Include a concise description of the device, specifying its name, model number, and intended use, which is crucial for contextualizing the entry.
- Submission Type: Clearly indicate that the entry is a 510(k) application, and include the relevant submission number if applicable to provide a clear reference for FDA reviewers.
- Regulatory Information: Reference any previous entries related to the device, including relevant K-numbers, to demonstrate the device's regulatory history and compliance.
- Summary of Changes: If applicable, provide a summary of any modifications made to the device compared to the previously cleared version, ensuring clarity on how it differs from existing products.
- Signature: The introductory document must contain the signature of an authorized representative, which confirms the accuracy and completeness of the information given, a critical aspect of regulatory filings.
- Clearly indicate the date of delivery at the end of the document to establish a timeline for FDA processing.
By incorporating these elements, manufacturers can ensure their application complies with FDA requirements, which can be illustrated by a 510k cover letter example, helping to mitigate common challenges encountered during the 510(k) submission process. For instance, the case study titled "Common 510(k) Challenges" highlights that manufacturers often struggle with a lack of understanding of the process, failure to demonstrate substantial equivalence, and insufficient documentation. Acknowledging these challenges highlights the significance of thoroughness in the application.
Furthermore, it is essential to recognize that a device must be 'substantially equivalent' to a legally marketed medical device, referred to as a 'predicate device,' which further emphasizes the significance of the 510(k) documentation in relation to FDA regulations. Experts like Ana Criado, Director of Regulatory Affairs at Mahu Pharma, and Katherine Ruiz, a professional in Regulatory Affairs for Medical Devices and In Vitro Diagnostics, stress the significance of understanding these components. Trey Thorsen, a regulatory consultant, emphasizes this importance in our interview, where he answers common questions regarding the 510(k) process.
Acknowledging these requirements not only streamlines the review process but also improves the chances of a successful entry.
Common Pitfalls in 510(k) Cover Letter Preparation
When preparing a 510(k) cover letter, it is crucial for applicants to recognize and avoid several common pitfalls that can jeopardize the success of their application:
-
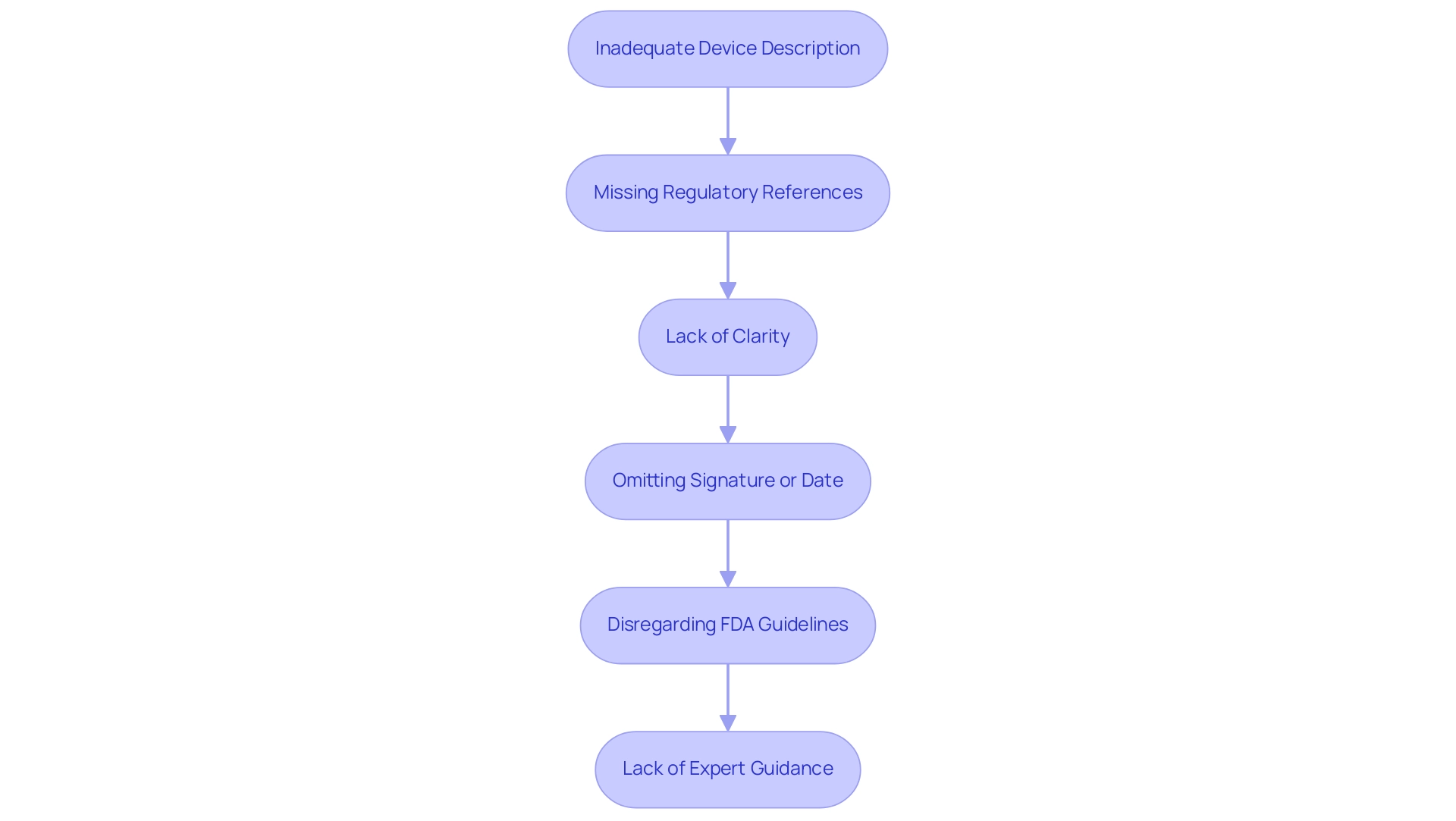
Inadequate Device Description: A failure to provide a clear and comprehensive description of the device can lead to misunderstandings during the review process, potentially resulting in delays or rejections. Understanding the device's intended use and comparing it with predicate devices can help clarify this description.
-
Missing Regulatory References: Neglecting to reference prior entries or existing K-numbers can hinder the FDA's ability to assess the proposal efficiently, as this essential context is critical for reviewers.
-
Lack of Clarity: The use of overly technical language or industry jargon can obscure the intended message, making it difficult for reviewers to grasp the fundamental aspects of the document. Jon Speer emphasizes,
FDA reviewers see probably dozens, maybe hundreds of entries in a finite period of time. Some are better than others. Well, make yours stand out. Make yours easier to read.
-
Omitting Signature or Date: Failing to include an authorized signature or the date of submission can lead to automatic rejection, as these elements confirm the legitimacy and timing of the submission.
-
Disregarding FDA Guidelines: Not following the FDA’s specific recommendations on submission format and content can cause significant delays. Recent news highlights that many FDA rejections stem from issues related to the 510(k) cover letter example formatting, underscoring the importance of compliance with established guidelines.
-
Lack of Expert Guidance: Engaging with on-demand FDA filing consultants, such as experts like Ana Criado, who have extensive experience in Regulatory Affairs, can provide significant value in navigating Regulatory processes. Ana has worked with global companies like General Electric and Omron Healthcare, allowing her to understand the nuances of the 510(k) process and the specific challenges applicants face. Her leadership in cannabis regulation further showcases her ability to navigate complex regulatory frameworks. By leveraging her expertise, applicants can avoid common pitfalls and ensure that their entries are well-prepared and compliant.
By proactively addressing these pitfalls and considering the importance of expert guidance, particularly from seasoned professionals like Ana Criado, applicants can significantly enhance the likelihood of a successful 510(k) application, ultimately streamlining the path to market for their medical devices.
The Importance of Clarity and Organization in Your Cover Letter
In the realm of 510(k) applications, clarity and organization are paramount. A well-structured 510k cover letter example enables reviewers to swiftly grasp essential information, which significantly expediting the evaluation process. This is especially significant considering the ongoing discussions about 'predicate creep,' which emphasizes the need for a strong methodology in regulatory applications.
To enhance clarity, consider the following best practices:
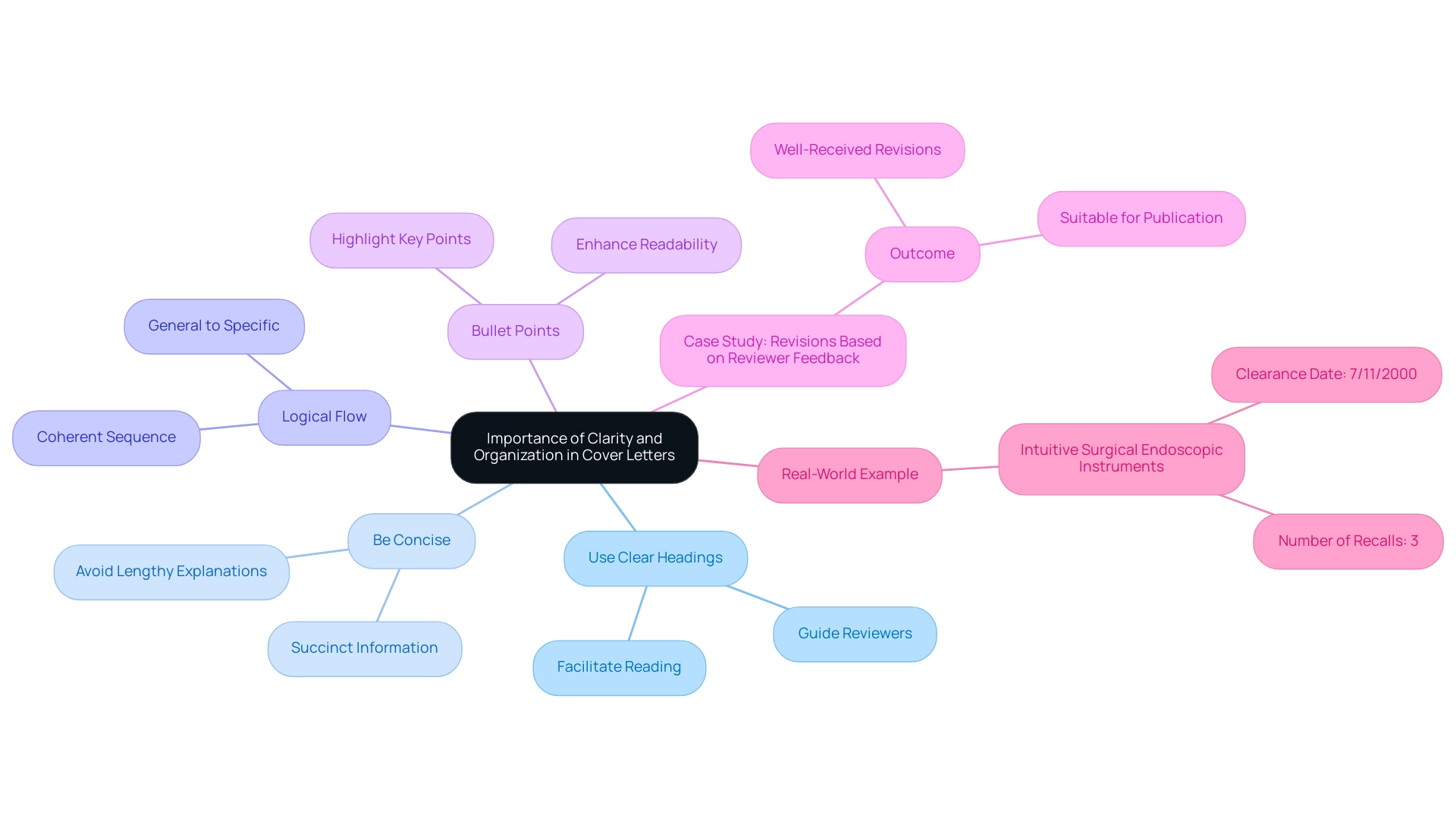
- Use Clear Headings: Clearly delineate sections with headings that guide reviewers through the content, facilitating a smoother reading experience.
- Be Concise: Focus on delivering succinct, relevant information rather than lengthy explanations, ensuring that all necessary details are communicated effectively.
- Logical Flow: Present information in a coherent sequence, beginning with general device information and progressing to specific regulatory details, which aids in comprehension.
- Bullet Points: Incorporate bullet points for lists, as they enhance readability and allow key points to stand out, making it easier for reviewers to identify critical elements at a glance.
For instance, the Intuitive Surgical Endoscopic Instruments received clearance on 7/11/2000 and experienced three recalls, underscoring the real-world implications of clarity and organization in documentation. By prioritizing clarity and organization, applicants can significantly enhance the effectiveness of their applications, including the use of a 510k cover letter example, making them not only user-friendly but also conducive to a more efficient review process. As noted by regulatory expert Quanzeng Wang, 'Thank you very much for the detailed and responsive edits; I appreciate the engagement with the reviewer & editorial feedback; and believe the manuscript will be an important contribution to the literature.'
This highlights the significance of clear communication in FDA applications, which is anticipated to be a crucial factor in 2024. Furthermore, the case study on revisions based on reviewer feedback illustrates how clarity and organization can lead to positive outcomes in the review process, reinforcing the necessity of these practices for future applications.
Understanding the Role of the Cover Letter in 510(k) Submissions
The introductory document is a crucial component of the 510(k) filing process, and it serves as a 510k cover letter example, offering important context for FDA evaluators. Its significance can be outlined through several key functions:
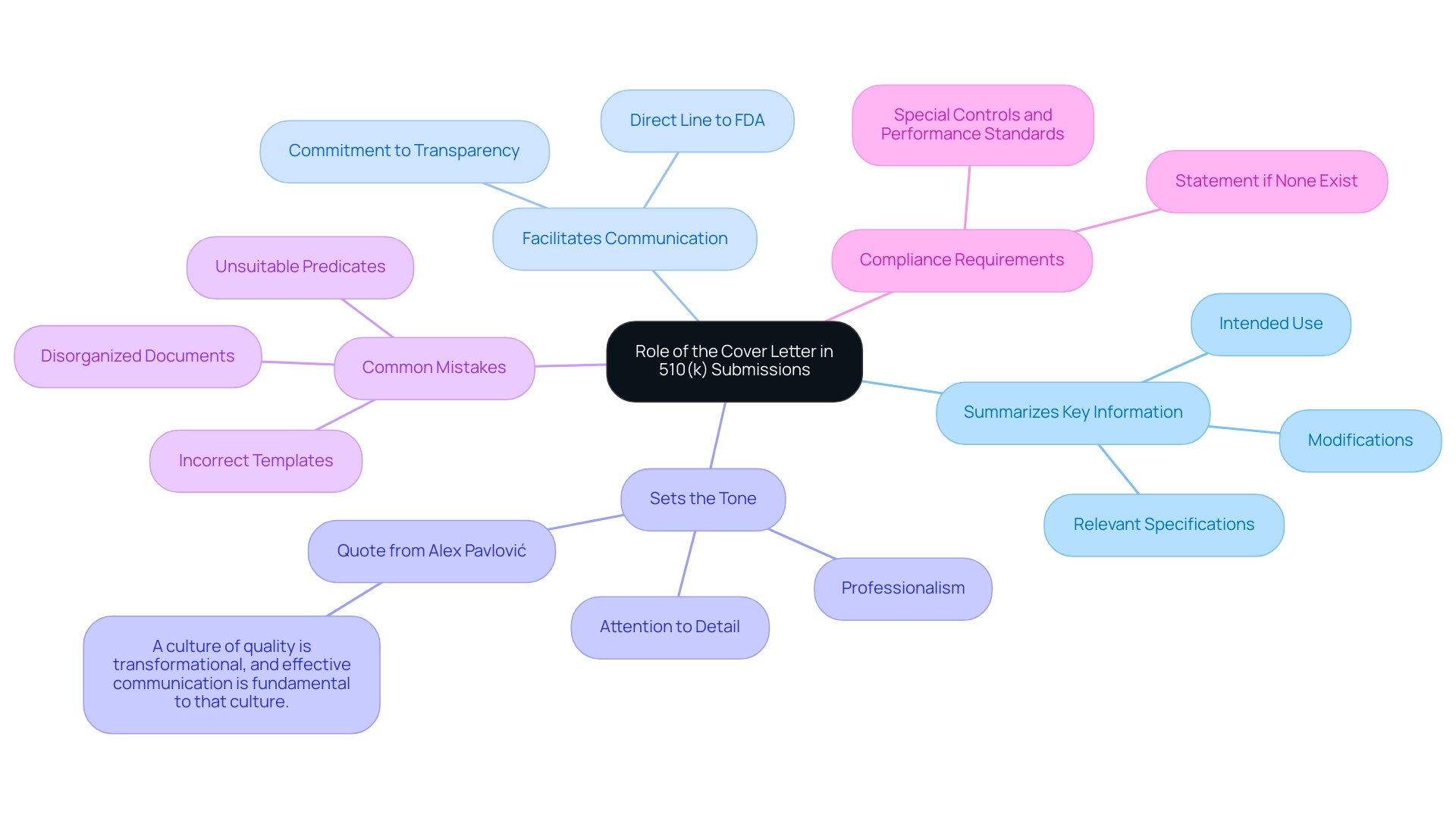
- Summarizes Key Information: The introductory document succinctly presents crucial details about the medical device, including its intended use, modifications, and relevant specifications, ensuring that reviewers have a clear understanding from the outset.
- Facilitates Communication: By establishing a direct line of communication with the FDA, the introductory document underscores the applicant's commitment to transparency, which can enhance the overall review experience.
- Sets the Tone: A meticulously crafted introductory document conveys professionalism and attention to detail, positively influencing the FDA's perception of the submission. As pointed out by quality and compliance specialist Alex Pavlović,
A culture of quality is transformational, and effective communication is fundamental to that culture,
highlighting that a well-organized application is a crucial element of this communication. Furthermore, adherence to specific controls and performance standards is vital, and the introduction should demonstrate this dedication to regulatory compliance. Grasping the complex function of the introductory document, particularly the 510k cover letter example, is crucial for avoiding frequent errors highlighted in case studies and ensuring a smooth and effective process. By avoiding pitfalls such as incorrect templates and disorganized documents, applicants can significantly improve review outcomes and expedite market access for devices.
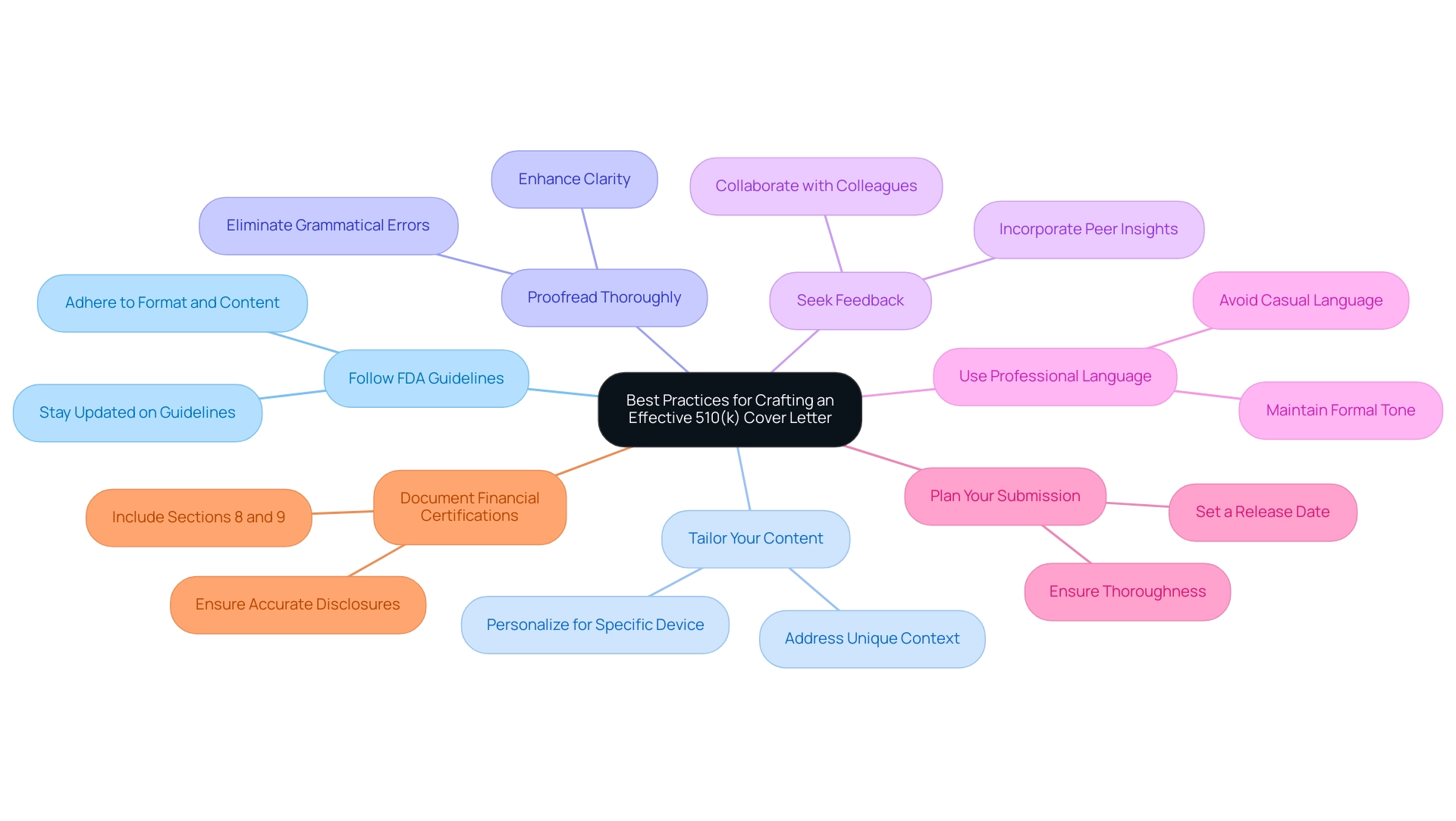
Best Practices for Crafting an Effective 510(k) Cover Letter
Creating a strong 510k cover letter example is essential for successful filings. To enhance your chances of approval, consider implementing the following best practices:
- Follow FDA Guidelines: It is essential to adhere strictly to the FDA's recommendations regarding format and content. Grasping the most current guidelines can significantly affect outcomes, as the majority of 510(k) applications are rejected on the first attempt due to insufficient compliance.
- Tailor Your Content: Personalize each message for its specific purpose, ensuring that it directly addresses the device in question and its unique context. This tailored approach demonstrates thoughtful consideration of the regulatory requirements and can greatly improve clarity.
- Proofread Thoroughly: Meticulous proofreading is necessary to eliminate grammatical errors and enhance clarity. A polished document reflects professionalism and attention to detail, which are critical in the highly regulated field of medical devices.
- Seek Feedback: Collaborating with colleagues for insights and suggestions can provide valuable perspectives. Peer evaluations can assist in recognizing areas for enhancement that may have been ignored, fostering a more comprehensive contribution.
- Use Professional Language: Maintain a formal tone throughout the letter, steering clear of casual language. This level of professionalism reinforces the seriousness of the document and aligns with the expectations of regulatory reviewers.
- Plan Your Submission: As one expert aptly stated, "In my opinion, you should pick a release date and try to stick with it unless there are serious issues that would lead to patient or business reputational harm." Such foresight can streamline your timeline for proposals while ensuring thoroughness.
- Document Financial Certifications: A real-world example is found in the case study related to Sections 8 and 9 of the FDA 510(k) application process, which involve financial certifications and declarations of conformity. Accurate financial disclosures and compliance declarations are essential for a complete and credible submission, thereby avoiding potential rejection.
By integrating these best practices into the preparation of your 510k cover letter example, you can significantly enhance its effectiveness, thereby increasing the likelihood of a successful approval process.
Conclusion
Crafting a compelling 510(k) cover letter is critical for facilitating a successful submission to the FDA. By incorporating essential components such as clear applicant and device information, regulatory references, and a concise summary of changes, manufacturers can lay a strong foundation for their application. Recognizing common pitfalls, such as inadequate device descriptions and failure to adhere to FDA guidelines, is equally important to avoid delays or rejections.
Moreover, the significance of clarity and organization cannot be overstated. A well-structured cover letter not only aids reviewers in quickly grasping vital information but also reflects the professionalism and diligence of the submitting organization. Best practices, including meticulous proofreading, tailored content, and seeking expert feedback, further enhance the quality of the submission, ensuring that it meets the high standards set by regulatory authorities.
In conclusion, the 510(k) cover letter is more than just a formality; it is a pivotal element that influences the overall review process. By understanding its role and adhering to established guidelines, manufacturers can significantly improve their chances of a successful submission, ultimately paving the way for timely market access for their medical devices. The effort invested in crafting an effective cover letter is an investment in the future success of the product and the organization.




