Introduction
In the intricate landscape of medical devices, the role of Instructions for Use (IFUs) cannot be overstated. These critical documents serve as a vital link between manufacturers and healthcare professionals, providing essential guidance for the safe and effective operation of medical technology. As hospitals increasingly grapple with the consequences of user errors and medication-related complications, the importance of clear and comprehensive IFUs becomes paramount.
This article delves into the multifaceted purpose of IFUs, outlining their key components, regulatory requirements, and best practices for ensuring clarity. Furthermore, it explores the significance of user feedback and ongoing training in enhancing the usability of these instructions, while also examining emerging trends that promise to reshape the future of IFU development.
Through this exploration, the article underscores the essential role of IFUs in promoting patient safety and improving healthcare outcomes.
Understanding the Purpose of Instructions for Use (IFU) in Medical Devices
IFU for medical devices are essential communication resources that connect manufacturers and end-users of medical products. Their primary function is to deliver clear, concise, and comprehensive guidance on the safe and effective operation, maintenance, and troubleshooting of devices. By meticulously detailing proper usage procedures, potential risks, and contraindications, the IFU for medical devices significantly minimizes the occurrence of user errors.
This is particularly crucial in light of recent findings that indicate hospital admissions due to avoidable drug-related morbidity account for between 3.0% and 3.74% of all adult non-obstetric, non-elective admissions. Such statistics emphasize the necessity for strict adherence to regulatory standards, which instructions for use support, ultimately improving patient safety. Moreover, IFU for medical devices are essential in educating healthcare professionals, equipping them with the crucial knowledge to operate equipment safely and confidently within clinical settings.
As emphasized by health and science writer Karen Blum, 'The clarity and effectiveness of IFU for medical devices can have a pronounced impact on patient outcomes,' making them a critical aspect of device utilization in healthcare settings. Additionally, ECRI emphasizes that 'they need help from policymakers and stakeholders to fend off and respond to ransomware attacks,' which underscores the importance of clear communication in instructions for use amidst broader healthcare challenges. The case study titled 'Challenges in Estimating Medication Error Burden' illustrates the complexities surrounding medication errors and highlights the necessity for effective IFU for medical devices to address these issues, further reinforcing the critical role of IFU for medical devices in improving patient safety.
Key Components of an Effective IFU: What You Must Include
An effective IFU for medical devices must encompass several essential components to deliver comprehensive guidance for users. These components typically include:
- Device Description: A precise explanation of the instrument's purpose and intended application.
- Instructions for Use: Detailed, step-by-step guidance on safely and effectively utilizing the equipment.
- Warnings and Precautions: Critical safety information designed to avert misuse and mitigate potential risks.
- Maintenance and Care: Specific instructions on cleaning, storing, and maintaining the equipment to ensure its longevity and optimal performance. Notably, cleaning instructions should specify safe chemicals and proper disassembly and reassembly procedures, which are crucial for safe operation.
- Troubleshooting: A guide to common issues users may face, paired with practical solutions.
- Regulatory Compliance Information: Affirmation that the device adheres to relevant regulations and standards, which is vital in the context of the evolving healthcare landscape. For example, compliance with instructions for use supports the transition to value-oriented care in the US healthcare system, as shown by the US Centers for Medicare & Medicaid Services declaring, "As the US healthcare system moves from fee-for-service models to a value-based care reimbursement model, healthcare environments must redirect their focus on conserving resources and delivering optimal patient care."
- Contact Information: Manufacturer details for user support and inquiries, facilitating prompt assistance when needed.
By incorporating these elements, manufacturers enhance the effectiveness of their instructions for use (ifu for medical devices), which promotes safe and effective utilization of the product and ultimately contributes to improved patient outcomes, such as the reduction of heart failure re-admission rates by as much as 78% for eligible Medicare patients using CardioMEMS technology, while ensuring compliance with regulatory standards.
Regulatory Requirements for IFUs: Navigating Compliance
Adhering to regulatory standards is crucial in the development of IFU for medical devices. In the United States, the Food and Drug Administration (FDA) mandates that all medical instruments must include an IFU for medical devices that adheres to the guidelines set forth in 21 CFR Part 801. These guidelines mandate that the IFUs for medical devices be composed in a clear and understandable way for the intended audience, including all essential information to ensure the safe and effective utilization of the product.
Studies indicate that a significant portion of users struggle with IFU readability, with statistics showing that out of a total of 220 subjects tested, 51 had the condition of interest, highlighting the critical need for clarity in these documents. In Europe, the Medical Device Regulation (MDR) enforces comparable requirements, compelling producers to offer detailed information about the item, including its intended purpose, usage instructions, safety details, and the IFU for medical devices. Regulatory Affairs experts such as Ana Criado, who serves as the Director of Regulatory Affairs at Mahu Pharma, and Katherine Ruiz, a specialist in medical devices and in vitro diagnostics, emphasize the importance of these regulations.
Criado's extensive experience at INVIMA, where she contributed to developing regulatory frameworks, and Ruiz's expertise in assisting foreign producers through the Colombian market clearance process, underscore the necessity of adhering to these standards. An illustrative case study titled 'Consequences of Using Resolver Results' reveals that revising original results using resolver outcomes can create unclear definitions of condition status and lead to incorrect calculations of sensitivity and specificity. The FDA recommends against such revisions, advocating for the reporting of original results and appropriate agreement measures instead.
By diligently following these regulations, producers not only achieve compliance but also bolster the credibility and reliability of their products in the marketplace. Recent FDA recommendations emphasize the importance of careful study planning when evaluating diagnostic tests, advocating for the use of a reference standard to accurately report diagnostic accuracy. This adherence to regulatory standards ultimately fosters trust among healthcare professionals and patients alike.
Best Practices for Writing Clear and Concise IFUs
To ensure the creation of clear and concise IFU for medical devices, manufacturers should implement the following best practices:
- Use Simple Language: Employ straightforward language that minimizes technical jargon, making the content accessible to the intended audience.
- Organize Information Logically: Structure the information in a coherent manner, utilizing headings, bullet points, and lists to enhance readability and facilitate quick understanding.
- Incorporate Visual Aids: Integrate diagrams, illustrations, and photographs to complement the text and help individuals in visualizing complex instructions. Research indicates that visual aids can significantly enhance comprehension, making them an essential component of effective IFUs.
- Test the IFU: Conduct usability testing with real participants to pinpoint areas of confusion, allowing for revisions that enhance clarity and usability.
- Update Regularly: Regularly review and update the IFU for medical devices to reflect changes in device design, usage, or regulatory requirements, ensuring that the instructions remain relevant and effective.
By adhering to these practices, manufacturers can substantially enhance the effectiveness of their IFUs for medical devices, ultimately leading to safer and more effective use of these products. This structured approach not only enhances customer satisfaction—potentially increasing it by 10%—but can also generate significant revenue growth, with the potential to add an incremental $10 million in revenue and reduce costs by 5%.
Furthermore, concise communication is crucial; as highlighted in a case study on web writing, paragraphs should ideally be limited to 70 words or less to facilitate quick information retrieval. This not only improves user satisfaction but also strengthens the competitive position within the sector.
The Role of User Feedback in Improving IFUs
User feedback serves as a cornerstone for the ongoing enhancement of IFU for medical devices in the sector. Manufacturers should proactively engage users—healthcare professionals and patients alike—to gather insights that reveal challenges or ambiguities within the IFU for medical devices. This engagement can be realized through diverse methodologies, such as:
- Surveys
- Focus groups
- Direct interviews
Analyzing the feedback obtained not only aids producers in identifying specific areas needing improvement but also fosters revisions that enhance clarity and usability. Collaboration among stakeholders, including producers, regulatory agencies, and healthcare professionals, is essential for refining these instructions. By creating a continuous feedback loop, manufacturers not only enhance the quality of their instructions but also confirm their dedication to consumer safety and satisfaction, ultimately resulting in better patient outcomes.
This emphasis on clearer IFU for medical devices is paramount, as studies highlight that discrepancies in cleaning protocols resulting from unclear instructions can elevate the risk of healthcare-associated infections. Moreover, companies are increasingly embracing an integrated feedback signal strategy to better comprehend customer experiences, which indicates a wider trend in the industry towards improving engagement. Thus, utilizing feedback from individuals greatly enhances medical equipment safety and effectiveness, reinforcing its essential value in the industry.
The significance of client satisfaction is further emphasized by the performance of top NPS leaders, such as USAA Insurance and Apple, who utilize customer feedback to gauge and enhance their services.
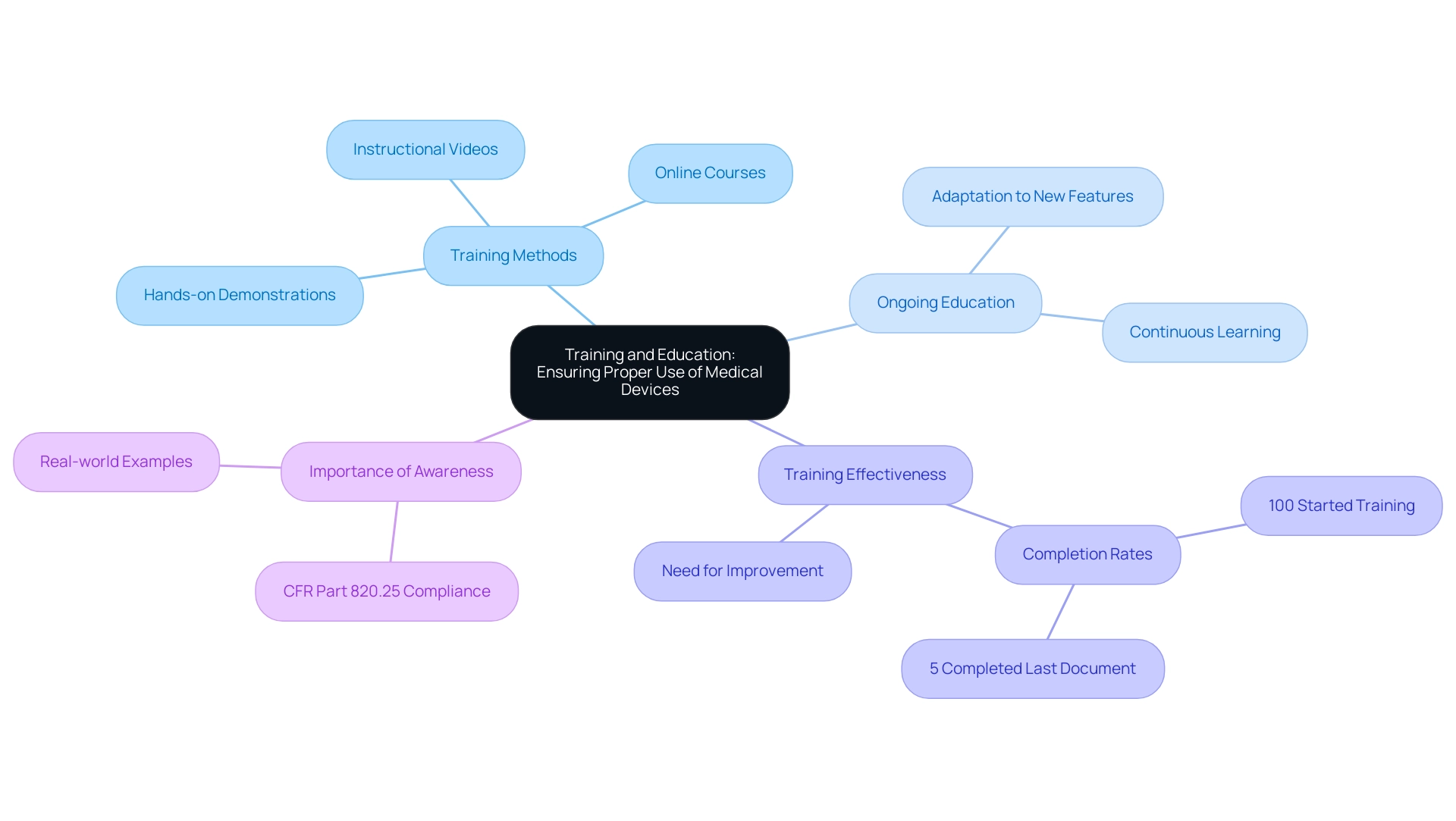
Training and Education: Ensuring Proper Use of Medical Devices
While the ifu for medical devices are crucial for the safe functioning of medical equipment, they must be backed by thorough training and educational programs for individuals. It is essential that producers introduce training programs that cover not only the operation of the equipment but also the related hazards and safety protocols. Effective training methodologies may include:
- Hands-on demonstrations
- Instructional videos
- Online courses
All designed to cater to different learning styles.
Furthermore, ongoing education is crucial, particularly as advancements or new features are introduced. Notably, recent statistics reveal that while 100% of the training team has engaged with a given activity, only 5% of trainees have successfully completed the last document within the training scope. This emphasizes a significant gap in training effectiveness, underscoring the need for producers to prioritize user education.
As stated in CFR Part 820.25, '(2) Personnel who perform verification and validation activities shall be made aware of defects and errors that may be encountered as part of their job functions.' This reinforces the importance of awareness in training programs. Additionally, the case study titled 'Impact of COVID-Related Lockdown on Mental Health in Germany' illustrates the critical need for effective training programs, as variations in mental health outcomes were observed due to differing policy implementations.
By concentrating on these training aspects and incorporating real-world examples, manufacturers can significantly enhance the proficiency of healthcare professionals and patients in using medical tools, ultimately improving safety and efficacy in clinical settings. Additionally, recent discussions in the article 'Real-world data: a brief review of the methods, applications, challenges and opportunities' emphasize the changing landscape of training and education in the medical field.
Future Trends in IFU Development for Medical Devices
The area of guidelines for medical products is experiencing considerable change, driven by swift technological progress and changing consumer expectations. A noteworthy trend is the rise of digital formats, particularly ifu for medical devices, which can be conveniently accessed through mobile devices or QR codes. These formats not only facilitate real-time updates but also enrich the experience by providing additional resources and enhancing comprehension.
Significantly, 69% of stakeholders view AI as essential for attracting and retaining clients, highlighting the significance of engagement in the context of digital instructions. The potential of augmented reality (AR) is particularly promising, as it enables users to engage with instructions in an immersive way, allowing for better visualization and understanding of complex processes. As Nic Newman observed, immersive technologies like VR are becoming increasingly relevant in various fields, including healthcare.
As regulatory agencies increasingly prioritize user-centered design, producers must embrace innovative strategies to ensure their ifu for medical devices adhere to elevated standards of clarity and usability. Furthermore, the ongoing integration of social shopping with news content illustrates how digital formats are evolving in other sectors, potentially offering insights into how the medical device industry might adapt similarly. By proactively adapting to these trends, manufacturers position themselves to meet the dynamic needs of users in the healthcare sector, ensuring they stay competitive in a rapidly changing environment.
Conclusion
The exploration of Instructions for Use (IFUs) in the medical device sector reveals their indispensable role in promoting patient safety and enhancing healthcare outcomes. By providing clear, concise, and comprehensive guidance, IFUs bridge the communication gap between manufacturers and healthcare professionals, significantly reducing the risk of user errors and medication-related complications. The article underscores the importance of incorporating essential components, adhering to regulatory requirements, and implementing best practices to ensure effective IFUs.
Moreover, the significance of user feedback and ongoing training cannot be overstated. Engaging healthcare professionals and patients in the feedback process allows manufacturers to refine their IFUs continuously, thereby bolstering user satisfaction and safety. As the industry evolves, embracing emerging trends such as digital formats and interactive technologies will be crucial for enhancing the usability of IFUs.
In conclusion, the commitment to developing clear and effective IFUs is vital for the healthcare sector. By prioritizing clarity, regulatory compliance, and user engagement, manufacturers can not only improve the operation of medical devices but also foster a safer environment for patients. Ultimately, the focus on high-quality IFUs will lead to better healthcare outcomes, reinforcing the critical nature of these documents in the medical landscape.
Frequently Asked Questions
What is the purpose of Instructions for Use (IFU) for medical devices?
IFUs are essential communication resources that provide clear, concise, and comprehensive guidance on the safe and effective operation, maintenance, and troubleshooting of medical devices, minimizing user errors.
Why are IFUs important for patient safety?
IFUs help reduce the occurrence of avoidable drug-related morbidity and hospital admissions by ensuring strict adherence to regulatory standards, thereby improving patient safety.
What are the key components of an effective IFU for medical devices?
An effective IFU should include a device description, instructions for use, warnings and precautions, maintenance and care instructions, troubleshooting guidance, regulatory compliance information, and contact information for user support.
How do regulatory standards influence the development of IFUs in the United States?
The FDA mandates that all medical devices include IFUs that are clear and understandable, ensuring they contain essential information for safe and effective use, as outlined in 21 CFR Part 801.
What best practices should manufacturers follow when creating IFUs?
Manufacturers should use simple language, organize information logically, incorporate visual aids, conduct usability testing, and regularly update the IFUs to enhance clarity and effectiveness.
How can user feedback improve IFUs for medical devices?
Engaging users through surveys, focus groups, and interviews helps manufacturers identify challenges and ambiguities in the IFUs, leading to revisions that enhance clarity and usability.
What role does training play in the effective use of medical devices?
Comprehensive training programs that cover equipment operation, hazards, and safety protocols are essential for ensuring users can effectively utilize medical devices while minimizing risks.
What trends are emerging in the development of IFUs for medical devices?
There is a shift towards digital formats for IFUs, which can be accessed via mobile devices or QR codes, and the integration of augmented reality (AR) to enhance user engagement and understanding of complex instructions.




