Introduction
Peru is rapidly becoming a pivotal hub for Medtech clinical research, characterized by its favorable regulatory landscape and an increasing pool of skilled professionals. As Medtech companies navigate the complexities of the Latin American market, they encounter both opportunities and challenges, from regulatory hurdles to the need for effective patient recruitment strategies.
The collaboration between innovative organizations, such as Greenlight Guru and bioaccess™, is instrumental in advancing clinical research and bridging gaps in the industry. With the Peruvian government actively streamlining approval processes and enhancing healthcare infrastructure, the nation presents a unique environment for conducting clinical trials that yield valuable insights into medical devices and technologies.
This article delves into the essential services offered by Medtech Contract Research Organizations (CROs) in Peru, the transformative role of technology in these services, the challenges faced by the sector, and the future trends that will shape the landscape of Medtech research in the country.
Overview of Medtech CRO Services in Peru
Peru has emerged as an important participant in the medical technology research field, driven by Medtech CRO Services Peru and an expanding group of skilled experts. However, medical technology firms in Latin America face challenges such as:
- Regulatory hurdles
- Language barriers
- Fragmented resources
These challenges can impede their progress. The nation’s strategic position in Latin America, along with its commitment to enhancing healthcare infrastructure, has made it an attractive location for Medtech CRO Services Peru and medical technology research.
Collaborations such as the one between Greenlight Guru and bioaccess™ are crucial in closing gaps in research and innovation, enabling medical technology firms to enhance their products more swiftly. The Peruvian government has implemented several initiatives to streamline the approval processes for clinical studies, further enhancing the country's desirability for international sponsors. With a diverse patient population and a range of healthcare facilities, Peru presents a unique opportunity for medical technology companies to conduct trials that can yield valuable insights into various medical devices and technologies.
Moreover, bioaccess® stands out as a leading provider of Medtech CRO Services Peru in Latin America, delivering cost-effective services and expert support for medical technology startups. By assisting with regulatory approvals, research site activation, and subject recruitment, bioaccess® helps navigate the complexities of research, ultimately contributing to improved patient outcomes.
Key Services Offered by Top Medtech CROs in Peru
Top Medtech CRO Services Peru provide a comprehensive array of services designed to meet the specific needs of clients in the medical device sector. The following key services are paramount in facilitating effective medical research:
-
Compliance Affairs: These organizations provide essential support in navigating the intricate oversight landscape of Medtech CRO Services Peru, ensuring adherence to both local and international guidelines. This knowledge is essential, especially considering the complex guidelines that define medical device evaluations. The medical technology sector in Peru is projected to grow by X% over the next five years, underscoring the increasing demand for regulatory expertise.
-
Site Management: An essential role of Medtech CRO Services Peru is the identification and oversight of research sites. They ensure that these sites meet rigorous standards and are adequately equipped to conduct research efficiently, addressing challenges such as disinterested clinical research sites.
-
Study Setup and Approval: Medtech CRO Services Peru assist in the study setup and approval processes, including obtaining necessary ethics committee approvals and health ministry clearances. This guidance is essential for ensuring that experiments begin smoothly and adhere to all compliance requirements. Effective patient recruitment strategies are essential for the success of research studies, and Medtech CRO Services Peru can play a crucial role in this process. CROs implement innovative approaches to recruit and retain participants, tackling the prolonged subject recruitment issue that many startups face. Dawn A. Lissy, president of an international consulting firm, remarked,
At this point, we’re all a little lost in translation, but saying the same thing,
highlighting the necessity for clear communication strategies in patient engagement. The integration of public and private sector activities, as discussed in a recent workshop, emphasizes the importance of collaboration in improving patient care and outcomes.
-
Data Management and Biostatistics: To ensure that clinical study results are both reliable and valid, CROs offer robust data management solutions and thorough statistical analyses as part of their Medtech CRO Services Peru. These services support the integrity of study findings, which are crucial for compliance submissions. Medtech CRO Services Peru include routine site visits performed by CROs to oversee adherence to study protocols and compliance standards, ensuring that studies are carried out according to established guidelines. This is particularly relevant as oversight scrutiny increases.
-
Import Permits and Nationalization: Medtech CRO Services Peru assists in the import permit process and nationalization of investigational devices, ensuring that all essential compliance hurdles are cleared before trials commence.
-
Training and Support: Comprehensive training programs for site staff are provided in Medtech CRO Services Peru to ensure they are well-versed in study protocols and best practices. This support is critical for maintaining high standards in medical research.
-
Post-Market Surveillance: After products have entered the market, Medtech CRO Services Peru assist clients in monitoring their performance, ensuring ongoing compliance and safety. This service is becoming ever more crucial as regulatory scrutiny heightens following launch.
Furthermore, the recent progress in medical technology, like the use of electromagnetic tracking sensors in medical devices, emphasizes current trends that contract research organizations in the medical field must keep up with to deliver pertinent services.
These services not only boost the efficiency of the research process but also greatly enhance the quality and integrity of the data gathered. By concentrating on these areas, medical research organizations play a crucial role in attaining improved patient outcomes and enhancing the standards of healthcare studies in Peru while positively influencing local economies through job creation and healthcare improvement. Contact us today to discover how we can assist with your clinical research needs and accelerate your medical device to market.
The Role of Technology in Medtech CRO Services
Technology is fundamentally transforming Medtech CRO Services Peru, especially through the implementation of advanced data management systems and electronic data capture (EDC) platforms. These innovations significantly streamline data collection and analysis processes, resulting in reduced timelines for study completion. Moreover, remote monitoring tools empower CROss to oversee study sites efficiently, minimizing the necessity for frequent on-site visits—ensuring compliance while conserving valuable time and resources.
The integration of artificial intelligence (AI) and machine learning algorithms further revolutionizes patient recruitment by enabling the rapid analysis of vast datasets to identify suitable candidates effectively. For instance, bioaccess® excels in managing various studies, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies (PMCF)
This demonstrates a commitment to accelerating medical device research in Latin America. Their partnership with GlobalCare Clinical Trials has resulted in an impressive over 50% decrease in recruitment duration and a retention rate surpassing 95%, highlighting the real-world effect of their extensive management services for studies.
These key metrics not only reflect the effectiveness of bioaccess®’s methodologies but also highlight their ability to enhance operational efficiency and generate high-quality data, ultimately leading to more successful clinical trial outcomes. As the landscape of Medtech CRO Services Peru continues to evolve, the impact of such services becomes increasingly critical, driving global health improvement and innovation in the field.
Challenges Faced by Medtech CROs in Peru
Despite the promising outlook for Medtech CRO Services Peru, there are still significant challenges that remain. A main issue is the inconsistency in governance standards across various areas, complicating the approval process for medical studies. This policy inconsistency can result in delays and higher expenses, with research showing that 40% of medical studies in Peru face considerable administrative delays.
Additionally, limited access to specific patient populations poses a substantial barrier to recruitment efforts, particularly for specialized studies that rely on targeted demographics. Katherine Ruiz, a Regulatory Affairs expert with extensive experience in obtaining market clearance for medical devices in Colombia, exemplifies the expertise needed to navigate these complexities. Her background at INVIMA and knowledge of compliance reviews further underscore the importance of having seasoned professionals in the field.
With more than 20 years of experience in Medtech, bioaccess® provides a tailored method for overseeing studies efficiently. The continuous evolution of technologies and methodologies in the industry necessitates ongoing training and development for research personnel, ensuring they remain adept at handling new challenges. As Infosys stated, "With our recent acquisition of product design and development firm, Kaleidoscope Innovation, we plan to redefine patient treatment and consumer health across the globe," highlighting the importance of innovative approaches in overcoming these hurdles.
Moreover, extensive research study management services, such as Medtech CRO Services Peru provided by bioaccess®, offer essential assistance in feasibility assessments, site selection, study setup, project management, and reporting. Creative solutions such as the 3D Module, which improves the presentation and visualization of research data, can also assist in tackling some of the challenges noted. Tackling these obstacles requires proactive approaches and strong cooperation among stakeholders to enable the successful implementation of research studies, ultimately enhancing patient care and public health initiatives.
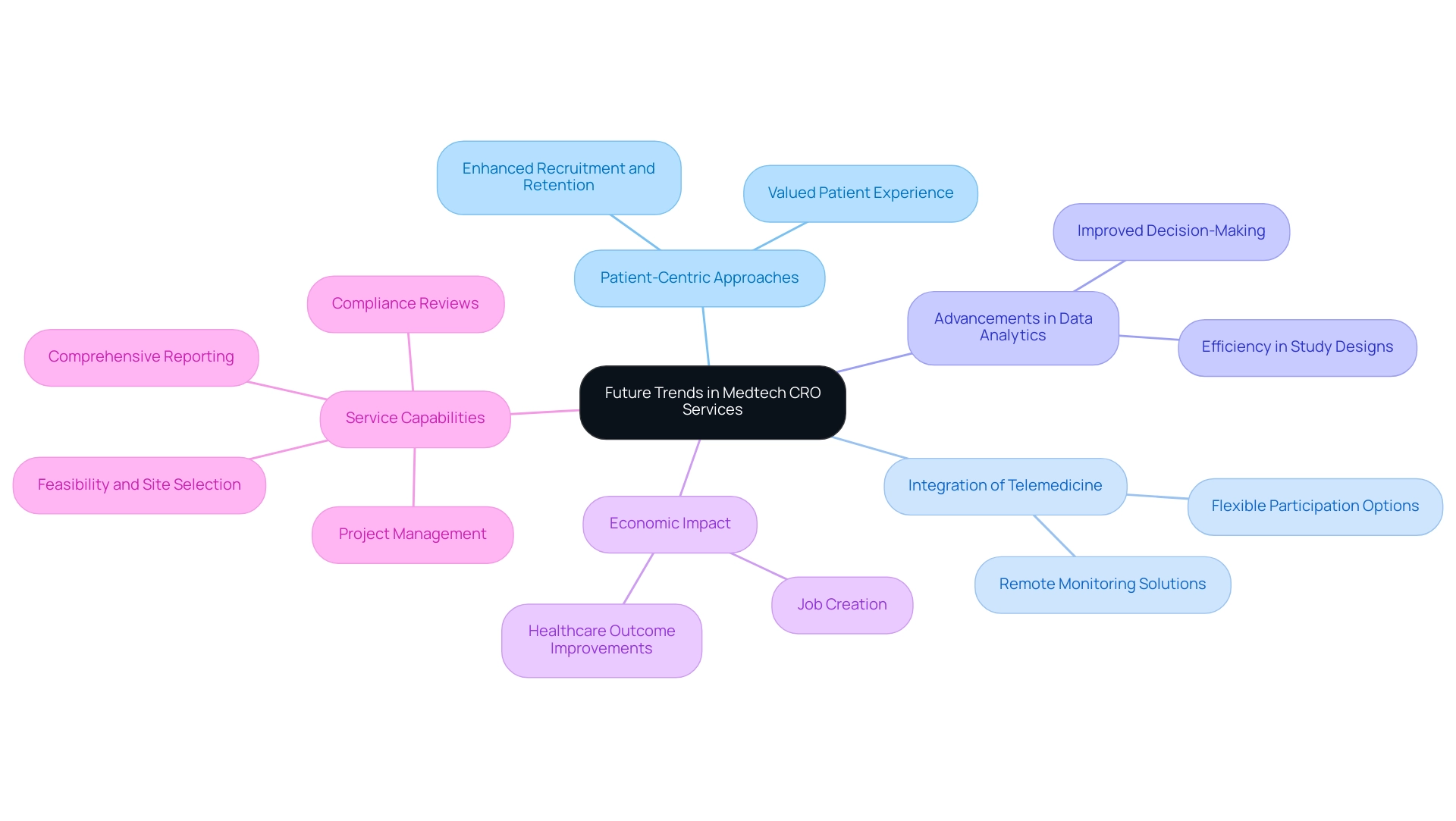
Future Trends in Medtech CRO Services
The medical technology sector is undergoing significant evolution, with several emerging trends poised to redefine Medtech CRO Services Peru. A primary focus is the increasing adoption of patient-centric approaches, prioritizing the patient experience at every stage of the clinical study process, which is likely to enhance recruitment and retention rates as participants feel more valued and supported. The expertise of organizations like bioaccess®, with over 20 years of experience in Medtech, in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies is crucial in this context.
Furthermore, the integration of telemedicine and remote monitoring solutions is set to expand, providing participants with flexible and accessible options for involvement, thus facilitating broader participation across diverse demographics. Furthermore, advancements in data analytics and artificial intelligence are anticipated to enhance the efficiency of study designs and improve decision-making processes. As highlighted by the Head of Data Management at George Clinical,
'With Zelta, we can support all areas of therapeutic studies.
We have a good mix of all phases of trials, from phase one to post-marketing surveillance studies, and have positioned for worldwide growth concluded studies with huge patient populations.'
This underscores the necessity for Medtech CRO Services Peru to remain dynamic and innovative in response to the evolving Medtech landscape. Moreover, the impact of medical studies on local economies is profound, creating jobs, promoting economic growth, and improving healthcare outcomes, which emphasizes the importance of international collaboration. A pertinent case study is ProTrials, which demonstrated the ability to perform a minor study change and fully document customer approval in just 30 minutes, exemplifying Zelta's capability to streamline processes in clinical research.
Our service capabilities include:
- Feasibility and selection of research sites and principal investigators
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Comprehensive reporting
This ensures a thorough and customized approach to meet client needs.
Conclusion
Peru's emergence as a key player in Medtech clinical research is underscored by its favorable regulatory environment, an expanding pool of skilled professionals, and a commitment to enhancing healthcare infrastructure. The collaboration between organizations like Greenlight Guru and bioaccess™ exemplifies how strategic partnerships can bridge gaps in clinical research, enabling Medtech companies to navigate the complexities of the Latin American market more effectively. The services provided by Medtech Contract Research Organizations (CROs) in Peru, including:
- Regulatory affairs
- Patient recruitment
- Data management
are essential in facilitating the successful execution of clinical trials and ultimately improving patient outcomes.
Despite the promising landscape, challenges such as regulatory variability and limited access to specific patient populations remain significant hurdles. However, the ongoing evolution of technology and innovative approaches within the Medtech sector offer a pathway to overcoming these challenges. The integration of advanced data management systems and patient-centric strategies will not only enhance operational efficiency but also ensure that trials are conducted with the highest standards of integrity and compliance.
Looking ahead, the Medtech CRO services in Peru are poised for transformation driven by trends such as:
- Telemedicine
- Artificial intelligence
- A focus on patient experience
These developments will be crucial in attracting international sponsors and fostering a collaborative environment that supports innovation and growth. As the industry continues to evolve, proactive strategies and robust partnerships will be essential in harnessing the full potential of Peru's Medtech research capabilities, ultimately contributing to improved healthcare outcomes and economic development in the region.
Frequently Asked Questions
What is the current status of Peru in the medical technology research field?
Peru has become an important player in medical technology research, driven by Medtech CRO Services Peru and a growing group of skilled professionals.
What challenges do medical technology firms in Latin America face?
The challenges include regulatory hurdles, language barriers, and fragmented resources, which can hinder their progress.
Why is Peru considered an attractive location for medical technology research?
Peru's strategic position in Latin America and its commitment to enhancing healthcare infrastructure make it appealing for Medtech CRO Services and medical technology research.
How do collaborations benefit medical technology firms in Peru?
Collaborations, such as the one between Greenlight Guru and bioaccess™, help close gaps in research and innovation, allowing firms to enhance their products more rapidly.
What initiatives has the Peruvian government implemented to support clinical studies?
The government has introduced initiatives to streamline the approval processes for clinical studies, increasing the country's attractiveness to international sponsors.
What services do top Medtech CRO Services Peru provide?
They offer services such as compliance affairs, site management, study setup and approval, data management and biostatistics, import permits and nationalization, training and support, and post-market surveillance.
How do Medtech CRO Services Peru assist with compliance?
They help navigate the complex regulations governing medical devices, ensuring adherence to local and international guidelines.
What role does data management play in Medtech CRO Services Peru?
CROs provide robust data management solutions and statistical analyses to ensure the reliability and validity of clinical study results.
What is the significance of training and support provided by Medtech CRO Services Peru?
Training ensures that site staff are knowledgeable about study protocols and best practices, which is crucial for maintaining high research standards.
How is technology transforming Medtech CRO Services Peru?
Advanced data management systems, electronic data capture platforms, and remote monitoring tools are streamlining data collection and analysis, reducing study timelines.
What are some key metrics demonstrating the effectiveness of bioaccess® in managing studies?
Bioaccess® has achieved over a 50% decrease in recruitment duration and a retention rate exceeding 95%, showcasing their operational efficiency.
What ongoing challenges does the medical technology sector in Peru face?
Challenges include inconsistent governance standards, administrative delays in study approvals, and limited access to specific patient populations for recruitment.
How does the evolution of technologies impact Medtech CRO Services Peru?
Continuous advancements necessitate ongoing training for research personnel to handle new challenges effectively.
What emerging trends are influencing Medtech CRO Services Peru?
Trends include adopting patient-centric approaches, integrating telemedicine, and utilizing data analytics and AI to enhance study efficiency and decision-making.
What are the potential economic impacts of medical studies in Peru?
Medical studies can create jobs, promote economic growth, and improve healthcare outcomes, highlighting the importance of international collaboration.




