Overview
Top Latin American countries for clinical research prominently include:
- Brazil
- Mexico
- Argentina
- Colombia
- Chile
Each of these nations presents unique advantages, such as diverse patient populations and cost-effective study environments. As the demand for clinical trials rises, these countries are becoming increasingly attractive due to their lower costs and evolving regulatory frameworks. Notably, initiatives like Colombia's R&D tax incentives significantly enhance the feasibility and appeal of conducting medical studies in the region. This shift underscores the importance of collaboration and strategic partnerships in advancing clinical research initiatives.
Introduction
Latin America is swiftly positioning itself as a dynamic and appealing destination for clinical research, driven by a distinctive combination of advantages that meet the evolving demands of the medical technology industry. The region's rich tapestry of ethnically diverse patient populations provides invaluable insights that enhance research applicability across various demographics.
In addition, the significantly lower costs associated with conducting clinical trials compared to North America and Europe make Latin America a compelling option for Medtech companies seeking efficient and effective research environments.
Recent regulatory advancements and supportive initiatives in countries like Colombia further strengthen the region's appeal, establishing it as a focal point for innovative clinical studies.
As the landscape continues to evolve, grasping the key players, regulatory frameworks, and patient recruitment strategies will be essential for harnessing the full potential of clinical trials in this vibrant region.
Why Latin America is an Attractive Destination for Clinical Research
Latin regions, particularly the leading Latin American countries for clinical research, have emerged as a pivotal center for medical studies, driven by a unique blend of advantages. A primary benefit is the region's ethnically diverse patient population, which is essential for gathering comprehensive data across various demographics. This diversity not only enriches research findings but also enhances the applicability of medical devices across different populations.
Moreover, the cost of conducting research studies in Latin America is significantly lower than in North America and Europe. For example, while the average cost for a complete trial in the U.S. can range from $30 to $50 million, with an estimated cost of $36,500 per participant, Latin American countries offer a more economical alternative, making it an attractive option for many Medtech companies. This cost-effectiveness is further bolstered by the evolving regulatory landscape, where numerous nations are actively refining their processes to foster a more supportive environment for medical studies.
A notable illustration of this trend is Colombia, which has enacted generous R&D tax incentives aimed at encouraging investment in innovation and development, particularly for small and midsize companies. These incentives are expected to stimulate growth in the healthcare sector, positioning Colombia as a more appealing location for trials and promoting innovation in the medical technology field. Such measures not only enhance the investment climate but also directly benefit Medtech companies seeking to conduct research in the region.
In collaboration with bioaccess™, Flow-FX has selected Colombia for a first-in-human trial of its innovative Flow-Screw medical device for delivering intraosseous antibiotics. This partnership exemplifies the strategic advantages of conducting research studies in Colombia, where bioaccess™ provides comprehensive management services, including feasibility assessments, site selection, compliance evaluations, project oversight, and reporting.
Industry leaders acknowledge these benefits. For instance, Patricio Ledesma, Head of Clinical Operations and Founder at Sofpromed CRO, emphasizes the significance of Latin regions in the global landscape of trials, noting its capability to assist biotech firms in effectively conducting phase I-IV studies. He states, "Latin regions provide a distinctive opportunity for research studies, considering its varied patient demographics and cost benefits."
Furthermore, the impact of medical studies extends beyond immediate outcomes; it is anticipated to benefit local economies by generating employment, enhancing healthcare services, and fostering international cooperation in the medical technology sector.
In conclusion, the combination of a diverse patient population, reduced study costs, and an evolving regulatory landscape positions the top Latin American countries for clinical research as increasingly attractive locations for studies in 2025. This region not only offers strategic advantages for Medtech firms aiming to expedite their development processes but also contributes to the advancement of medical devices that can significantly improve patient outcomes.
Leading Latin American Countries for Clinical Trials
The top Latin American countries for clinical research are Brazil, Mexico, Argentina, Colombia, and Chile, each contributing uniquely to the region's medical studies. Brazil leads the group with nearly 10,000 studies registered as of 2024, reinforcing its status as the largest market in the area. This robust infrastructure is complemented by a diverse population, enhancing the applicability of research results across various demographics.
Significantly, involvement in medical studies in Latin America increased from 6.04% in 2015 to 8.54% in 2021, indicating a rising commitment to scientific exploration.
Mexico stands out as a formidable competitor, leveraging its large population and an expanding array of medical study facilities. The growth of these centers is crucial, as they enable a greater number of studies and attract global sponsors seeking effective study implementation.
Argentina has emerged as a notable participant, demonstrating swift progress in study capabilities. The nation is recognized for its innovative methods and strong dedication to enhancing study quality, making it an appealing location for medical investigations.
Colombia is gaining traction as a competitive option, particularly due to its cost-effective examination solutions and a solid regulatory framework that facilitates efficient study approvals. Recent partnerships, such as the one between bioaccess™ and Caribbean Health Group, aim to position Barranquilla as the most attractive destination for research in Latin America. Supported by Colombia's Minister of Health, these initiatives are expected to significantly enhance the research landscape.
bioaccess™ offers extensive research study management services, including feasibility analysis, site selection, compliance evaluations, setup, import permits, project management, and reporting. Projections indicate that Colombia could facilitate over 100 new studies annually, potentially generating close to $500 million in economic benefits each year. This economic impact underscores the importance of ongoing investment in investigative capabilities, as highlighted in the case study on scientific output in oncology studies, which emphasizes the region's need for increased funding.
Chile rounds out the top five, providing a favorable environment for experimental studies, particularly in oncology and medical devices. The nation's regulatory environment is advantageous, and its commitment to scientific inquiry is evident in the growing number of experiments being conducted. As Gustavo Werutsky noted, pharmaceutical firms have financed the majority of oncology studies in Latin countries, and additional funding will be essential from government or private foundations to sustain this growth.
As of 2025, the study landscape in the leading Latin American countries for clinical research, including Brazil, Mexico, Argentina, Colombia, and Chile, continues to evolve, fostering innovative advancements in the Medtech sector. The involvement of diverse populations in research is critical, as it enhances the relevance of results for new cancer therapies and other medical progress.
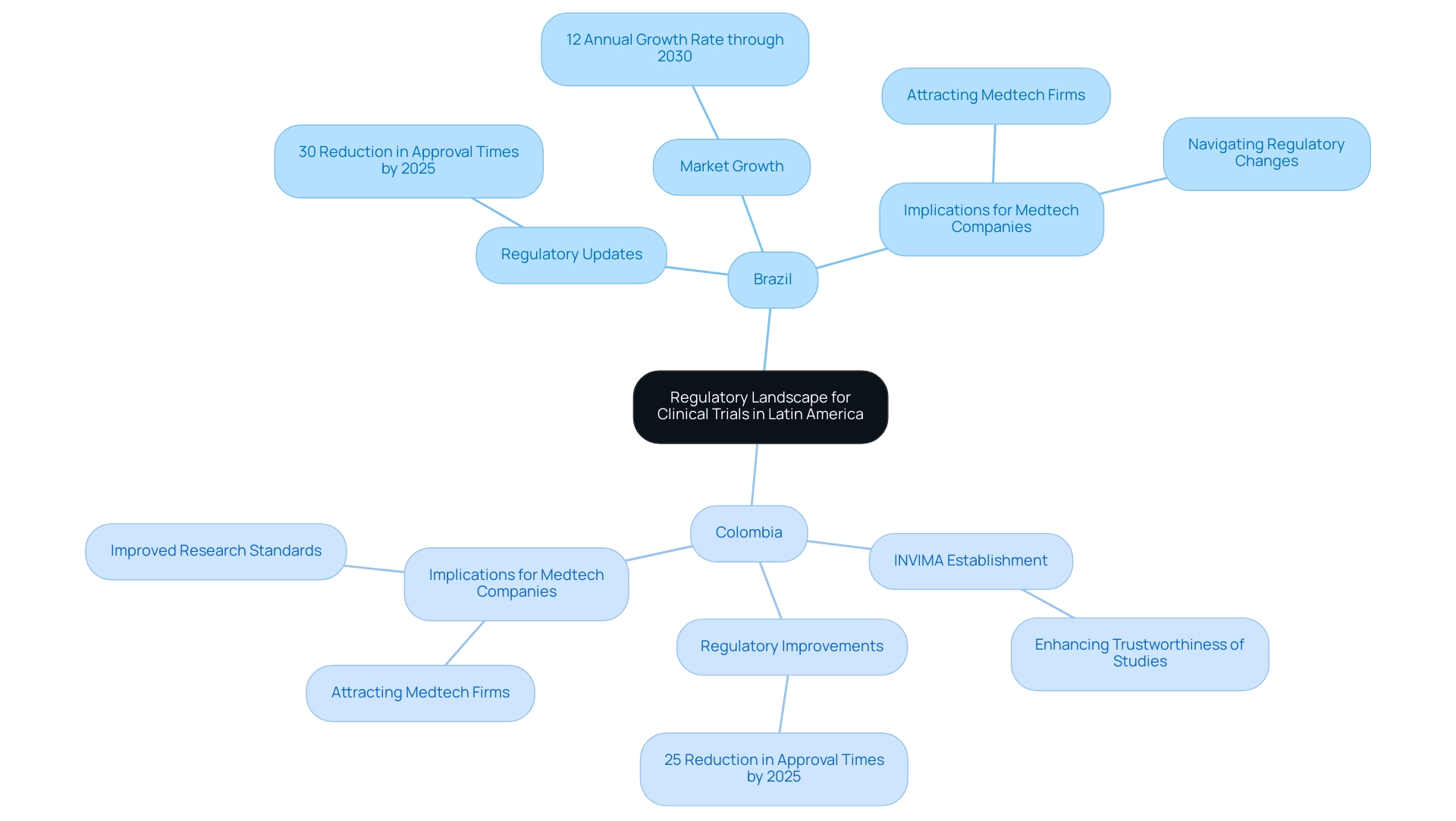
Navigating the Regulatory Landscape for Clinical Trials in Latin America
Navigating the regulatory landscape in the leading Latin American countries for clinical research presents unique challenges, as each nation enforces its own distinct set of rules and guidelines. However, significant progress is being made to simplify these processes. For instance, Brazil has recently implemented updates to its research regulations aimed at expediting approval times, which are expected to improve greatly by 2025, with projections suggesting a decrease in approval times by as much as 30%. This is particularly pertinent considering that the Brazil research market databook predicts a robust growth trajectory through 2030, with an anticipated annual growth rate of 12%, indicating a favorable environment for medical research.
In Colombia, the establishment of INVIMA as a level-4 regulatory agency marks a crucial advancement in upholding high standards for medical research. This agency not only supervises adherence but also enhances the trustworthiness of studies conducted within the country. By 2025, regulatory approval times in Colombia are expected to align more closely with international standards, with anticipated reductions of up to 25%, further attracting Medtech companies to the region. For example, INVIMA has effectively optimized the approval procedure for several high-profile research studies, demonstrating its positive impact on the industry.
Understanding these regulations is essential for Medtech companies, as adherence not only facilitates smoother research processes but also fosters confidence among local stakeholders and regulatory authorities. Recent case studies from the Horizon Databook illustrate how companies that proactively adapt to these regulatory changes in Brazil have successfully navigated the complexities of trials, positioning themselves advantageously in the competitive landscape. Moreover, the informed consent form (ICF) requirements, which mandate that documents be available in the participant's language and include signatures from both the participant and investigator, underscore the importance of transparency and ethical standards in scientific studies.
Additionally, biobanks must ensure that their informed consent forms encompass details about data access, rights to stored materials, and the potential for future research use, further highlighting ethical considerations. As the regulatory frameworks continue to evolve, Medtech companies must remain informed about the latest guidelines and best practices in Brazil and Colombia to capitalize on the opportunities presented by these dynamic markets. With bioaccess paving the way for medical device research assessments in Latin regions, companies can benefit from extensive research management services, including feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting. Colombia is recognized as one of the premier Latin American countries for clinical research, offering substantial advantages for conducting medical studies, including cost reductions of over 30% relative to the United States, expedited approval procedures, and a high-quality healthcare system, rendering it an attractive location for Medtech firms.
Patient Recruitment and Demographics: Key Considerations in Latin America
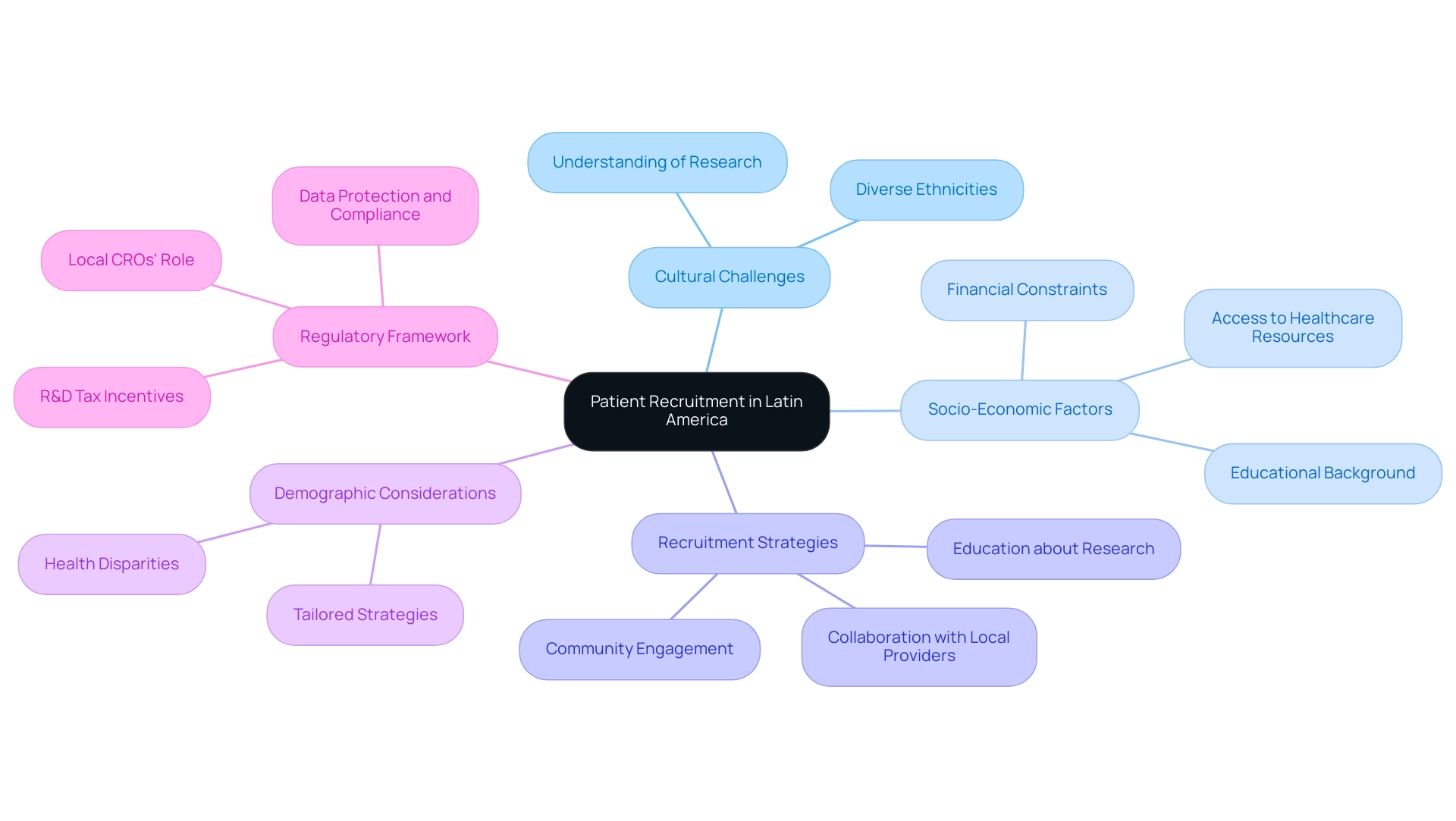
The unique blend of challenges and opportunities in patient recruitment is particularly evident in the leading Latin American countries for clinical research. This region's diverse population, encompassing a wide array of ethnicities and health conditions, offers a rich pool of potential participants for research studies. However, cultural differences and varying levels of understanding regarding research studies can complicate recruitment efforts.
Many individuals may not fully comprehend the benefits or processes involved in participating in research studies, which can hinder their willingness to engage.
To enhance recruitment success, implementing strategies such as community involvement and education about research studies is essential. Collaborating with local healthcare providers can also foster trust and improve outreach efforts. By leveraging existing relationships within communities, researchers can cultivate a more informed participant base, ultimately leading to higher enrollment rates.
Moreover, understanding the socio-economic factors that influence patient participation is crucial. For instance, access to healthcare resources, financial constraints, and educational background can significantly impact an individual's decision to engage in a study. Tailoring recruitment strategies to address these factors can facilitate more effective engagement.
Recent insights from research specialists highlight the importance of demographic considerations in recruitment strategies. The Hispanic Community Health Study underscores the necessity of recognizing health disparities, which is vital for effective patient recruitment strategies. By analyzing demographic data of study participants in the top Latin American countries for clinical research, researchers can identify patterns and adjust their strategies accordingly.
This data-driven perspective not only enhances recruitment initiatives but also ensures that studies accurately represent the groups they aim to assist.
Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, remarked, "My experience with bioaccess® during the initial human study in Colombia illustrated the effectiveness of local CROss in navigating the regulatory framework." This statement emphasizes the role of local organizations in facilitating successful recruitment and compliance, particularly in a landscape where Colombia's healthcare system is recognized for its quality and efficiency.
Furthermore, the partnership between bioaccess™ and Caribbean Health Group to establish Barranquilla as a prominent site for medical studies, supported by Colombia's Minister of Health, highlights strategic initiatives to enhance the health investigation landscape. Colombia is acknowledged as one of the top Latin American countries for clinical research, offering competitive advantages for first-in-human studies, including cost efficiency, regulatory speed, and high-quality healthcare. The case study titled 'Future Perspectives on Clinical Trials in Latin' stresses the need for improved infrastructure and collaboration among stakeholders to optimize resources in medical research.
Addressing data protection and grievance handling is essential for building trust and ensuring compliance in medical studies, ultimately boosting recruitment rates.
Additionally, the R&D tax and financial incentives available in Colombia, such as a 100% tax deduction for investments in science, technology, and innovation projects, significantly benefit medical device companies and can enhance recruitment efforts by making participation more attractive.
In summary, while patient recruitment in the leading Latin American countries for clinical research presents unique challenges, it also offers substantial opportunities for innovative strategies that can lead to successful studies. By prioritizing community engagement, education, and a nuanced understanding of socio-economic factors, researchers can effectively navigate the complexities of this diverse region.
Challenges and Solutions for Conducting Clinical Trials in Latin America
Conducting medical studies in leading Latin American countries for clinical research presents a unique set of challenges that require meticulous navigation. Among the primary issues are:
- Regulatory complexities
- Inconsistent infrastructure
- Patient recruitment difficulties
Colombia stands out as a top contender for clinical research in Latin America, offering significant advantages such as:
- Cost efficiency
- Expedited regulatory processes
- High-quality healthcare
For instance, the recent collaboration between bioaccess™ and Caribbean Health Group aims to position Barranquilla as a premier hub for medical research in the region, supported by Colombia's Minister of Health. This initiative exemplifies ongoing efforts to improve the regulatory environment and attract a greater number of clinical studies to the area.
Colombia boasts cost savings exceeding 30% compared to studies conducted in North America or Western Europe, with regulatory review timelines for IRB/EC and MoH (INVIMA) averaging just 90-120 days. Partnering with local contract research organizations (CROs) like bioaccess™ is essential, as they provide critical insights into the regulatory landscape and facilitate smoother study execution. A case study that emphasizes the importance of addressing language barriers demonstrates how meticulous translation of regulatory and patient-related materials can reduce delays in ethics committee approvals and enhance informed consent processes.
This approach not only improves the quality of trials but also ensures ethical treatment and safety for participants.
Moreover, cultural differences significantly impact informed consent processes in Latin regions, where patients may consent to participate in research studies without discussing treatment alternatives with their physicians. Investing in community outreach and educational initiatives can greatly enhance patient recruitment and retention rates. By cultivating trust and understanding within local communities, organizations can improve participation in research studies.
As highlighted by industry leaders such as Julio G. Martinez-Clark:
- "The government of Colombia appears to position itself among the top Latin American countries for clinical research, with a proactive effort to attract more research studies as part of its strategy to become a knowledge economy by 2031."
Additionally, R&D tax incentives, including a 100% tax deduction for investments in science, technology, and innovation initiatives, further enhance Colombia's attractiveness for research studies. Overcoming these challenges is vital for advancing medical studies in the region.
Furthermore, LACOG's initiatives to enhance access to cancer research and increase physician involvement in studies underscore the importance of collaboration and local participation. By proactively addressing regulatory complexities and leveraging local expertise, organizations can effectively navigate the clinical trial landscape in Latin America, ultimately contributing to the advancement of medical technology.
Conclusion
Latin America is emerging as a vital hub for clinical research, presenting a compelling combination of diverse patient populations, lower trial costs, and evolving regulatory frameworks. Key players such as Brazil, Mexico, Argentina, Colombia, and Chile offer unique advantages that attract Medtech companies, enabling impactful research at a fraction of the cost compared to North America and Europe.
Colombia, in particular, is gaining traction through government initiatives like R&D tax incentives and streamlined approval processes, making it an appealing destination for innovative studies. Collaborations between local organizations and international sponsors are essential for navigating the complexities of clinical trials, ensuring ethical standards, and maximizing operational efficiency.
While challenges such as regulatory intricacies and patient recruitment persist, effective strategies focused on community engagement and education can enhance participation rates. Understanding socio-economic factors and leveraging local expertise will be crucial in overcoming these hurdles.
In summary, Latin America's strengths position it as an attractive region for clinical research. By addressing existing challenges and capitalizing on its advantages, stakeholders can unlock the region's potential, leading to significant advancements in medical technology and improved patient outcomes. The future of clinical research in Latin America holds promise for substantial contributions to global health and innovation.
Frequently Asked Questions
Why are Latin American countries important for clinical research?
Latin American countries are important for clinical research due to their ethnically diverse patient populations, which provide comprehensive data across various demographics, enriching research findings and enhancing the applicability of medical devices.
How do the costs of conducting clinical research in Latin America compare to North America and Europe?
The cost of conducting research studies in Latin America is significantly lower than in North America and Europe. For example, a complete trial in the U.S. can cost between $30 to $50 million, while Latin American countries offer a more economical alternative.
What regulatory advantages do Latin American countries provide for clinical research?
Many Latin American nations are actively refining their regulatory processes to create a more supportive environment for medical studies, which enhances the investment climate for Medtech companies.
What specific incentives does Colombia offer to encourage clinical research?
Colombia has enacted generous R&D tax incentives to encourage investment in innovation and development, particularly for small and midsize companies, making it a more appealing location for clinical trials.
Can you provide an example of a clinical trial currently taking place in Colombia?
Flow-FX, in collaboration with bioaccess™, has selected Colombia for a first-in-human trial of its Flow-Screw medical device for delivering intraosseous antibiotics, showcasing the strategic advantages of conducting research in the country.
What are the top Latin American countries for clinical research?
The top Latin American countries for clinical research are Brazil, Mexico, Argentina, Colombia, and Chile, each contributing uniquely to the region's medical studies.
How has participation in medical studies in Latin America changed over recent years?
Participation in medical studies in Latin America increased from 6.04% in 2015 to 8.54% in 2021, indicating a rising commitment to scientific exploration in the region.
What role does Mexico play in clinical research in Latin America?
Mexico is a formidable competitor in clinical research due to its large population and expanding array of medical study facilities, which attract global sponsors seeking effective study implementation.
What advancements has Argentina made in clinical research?
Argentina has shown swift progress in study capabilities, recognized for its innovative methods and strong dedication to enhancing study quality, making it an appealing location for medical investigations.
What are Colombia's strengths in the context of clinical research?
Colombia offers cost-effective examination solutions and a solid regulatory framework that facilitates efficient study approvals, with projections indicating the potential for over 100 new studies annually.
How does Chile contribute to clinical research in the region?
Chile provides a favorable environment for experimental studies, particularly in oncology and medical devices, supported by a strong regulatory framework and a commitment to scientific inquiry.
What economic impact is expected from clinical research in Colombia?
Colombia's clinical research initiatives could generate close to $500 million in economic benefits each year, highlighting the importance of ongoing investment in investigative capabilities.




