Introduction
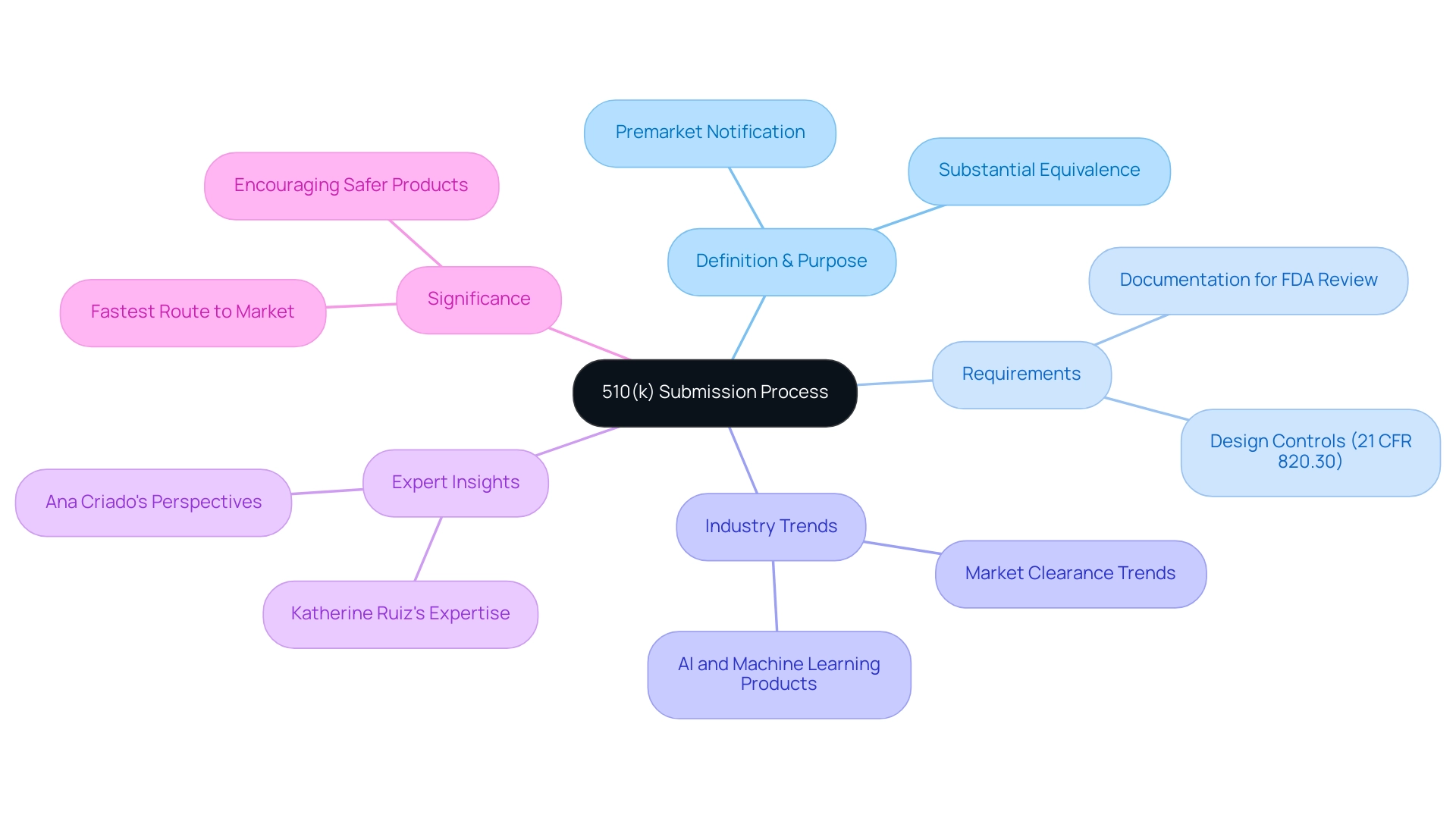
The 510(k) submission process plays a pivotal role in the regulatory framework for medical devices, serving as a key mechanism for manufacturers to demonstrate the safety and efficacy of their products. This premarket notification to the U.S. Food and Drug Administration (FDA) is particularly crucial for medium-risk devices, allowing for a streamlined pathway to market entry based on substantial equivalence to existing, legally marketed devices.
With the recent surge in artificial intelligence and machine learning technologies within the medical device sector, understanding the nuances of the 510(k) process has never been more essential. This article delves into the intricacies of 510(k) submissions, exploring:
- Eligibility criteria
- Preparation steps
- The FDA review process
While drawing on insights from leading regulatory experts to illuminate best practices for navigating this complex landscape.
What is a 510(k) Submission? An Overview for Medical Device Professionals
The 510(k) submission, which embodies the 510k meaning, acts as an essential premarket notification to the U.S. Food and Drug Administration (FDA), allowing medical product manufacturers to demonstrate that their offering is safe and effective based on substantial equivalence to an existing legally marketed item, referred to as a predicate item. This process is especially vital for Class II and III products, where adherence to design controls (21 CFR 820.30) is mandatory during development, and all manufacturers must have documentation available for FDA review. The streamlined nature of the 510(k) pathway illustrates the 510k meaning by allowing manufacturers to obtain market clearance without the extensive preclinical and clinical testing typically required for a Premarket Approval (PMA).
Consequently, this pathway frequently signifies the quickest and most economical route to market entry for medium-risk products, as emphasized in case studies. Recent trends indicate a significant increase in the number of AI and machine learning-enabled products cleared through the 510(k) process, with more AI/ML-enabled products cleared through this pathway than through De Novo classification, reflecting the FDA's focus on ensuring hardware safety while facilitating innovation. The FDA has noted,
The FDA believes use of these best practices outlined in this draft guidance, once finalized, will encourage the evolution of safer and more effective medical products in the 510(k) Program over time.
Grasping the 510k meaning and the complexities of the filing process is crucial for medical product professionals, including those mentored by experts like Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, who offers valuable perspectives from her extensive experience in regulatory consulting and biomedical engineering, including her work with international companies such as General Electric and Omron Healthcare. Additionally, Katherine Ruiz, an expert in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, offers further expertise in navigating these regulatory waters. Together, their insights highlight the significance of ensuring compliance with critical standards while facilitating quicker market access, especially considering the growing prevalence of AI and machine learning technologies in the medical landscape.
Devices Requiring 510(k) Submissions: Eligibility and Classification
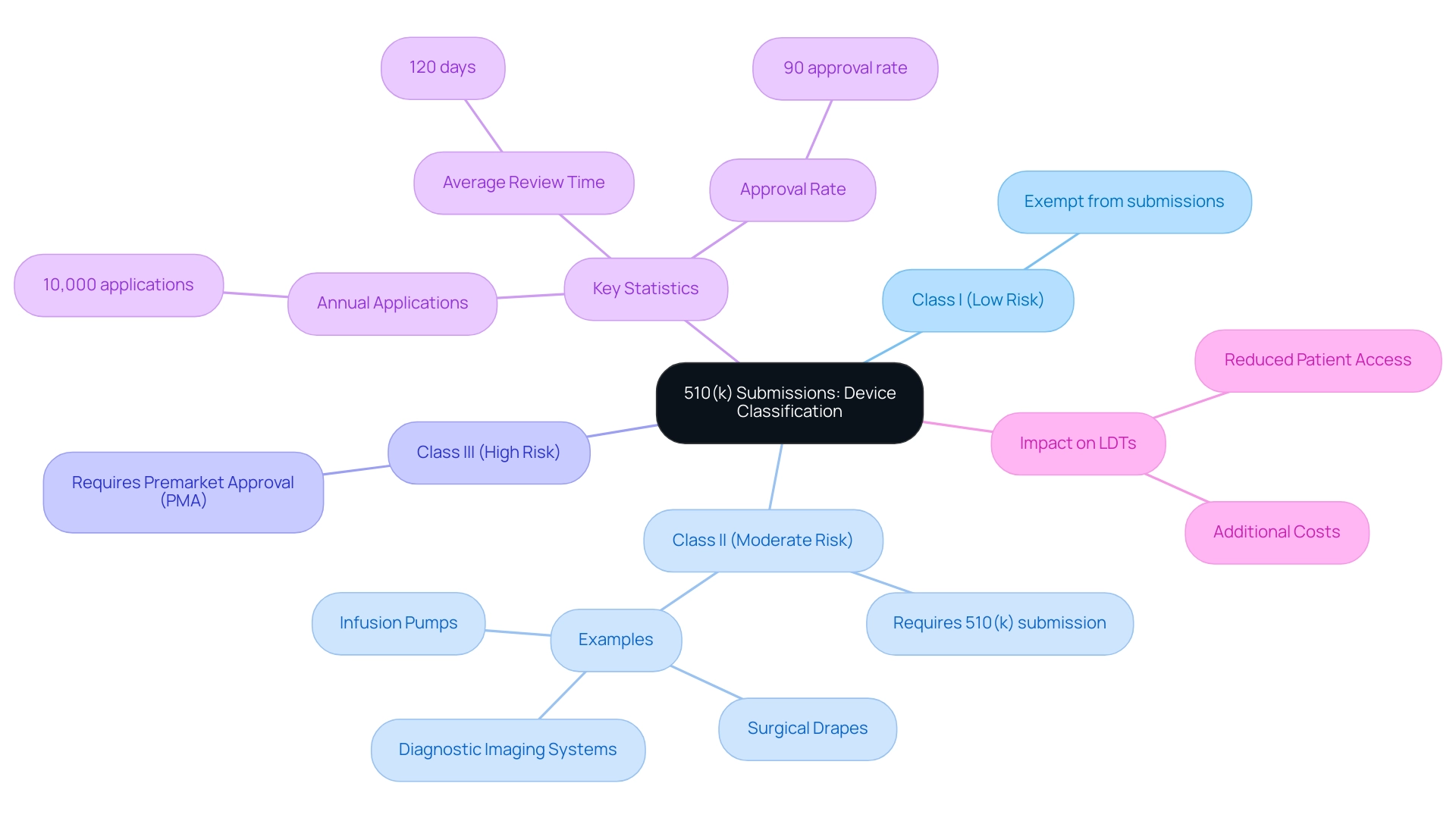
The 510(k) application is a critical element in the regulatory framework of medical instruments, highlighting the 510k meaning as it categorizes them into three primary groups based on their risk level:
- Class I (low risk)
- Class II (moderate risk)
- Class III (high risk)
While Class I products are generally exempt from submissions, understanding 510k meaning is essential for Class II items, which must typically submit a 510(k) to demonstrate substantial equivalence to an already approved predicate. Notable examples of Class II equipment include infusion pumps, surgical drapes, and diagnostic imaging systems.
Class III products, given their higher risk profile, usually necessitate a Premarket Approval (PMA) rather than a 510(k). Understanding these classifications is essential for clinical research professionals to avoid regulatory missteps. The FDA reports that approximately 10,000 applications are received annually, reflecting the 510k meaning with an impressive approval rate of around 90%.
This highlights the significance of the 510k meaning in the process of ensuring the safety and effectiveness of medical instruments. Furthermore, according to the FDA, the real-world evidence (RWE) generated through initiatives like the National Evaluation System for health Technologies (NEST) 'may be used not only for purposes of post market surveillance but also to support premarket regulatory decision-making and expanded indications for use after clearance or approval, among other things.' This highlights the evolving nature of regulatory understanding and decision-making.
Additionally, the imposition of additional costs associated with the 510k meaning process could limit the types and number of Laboratory Developed Tests (LDTs) available, ultimately reducing patient access to innovative diagnostic tests. Notably, Ana Criado, as the Director of Regulatory Affairs and an expert in this field, emphasizes the need for thorough comprehension of these regulatory processes. The case study titled '510(k) Submission Statistics' further illustrates this point, detailing the FDA's receipt of about 10,000 applications annually, which reinforces the 510k meaning process.
Preparing Your 510(k) Submission: Steps and Best Practices
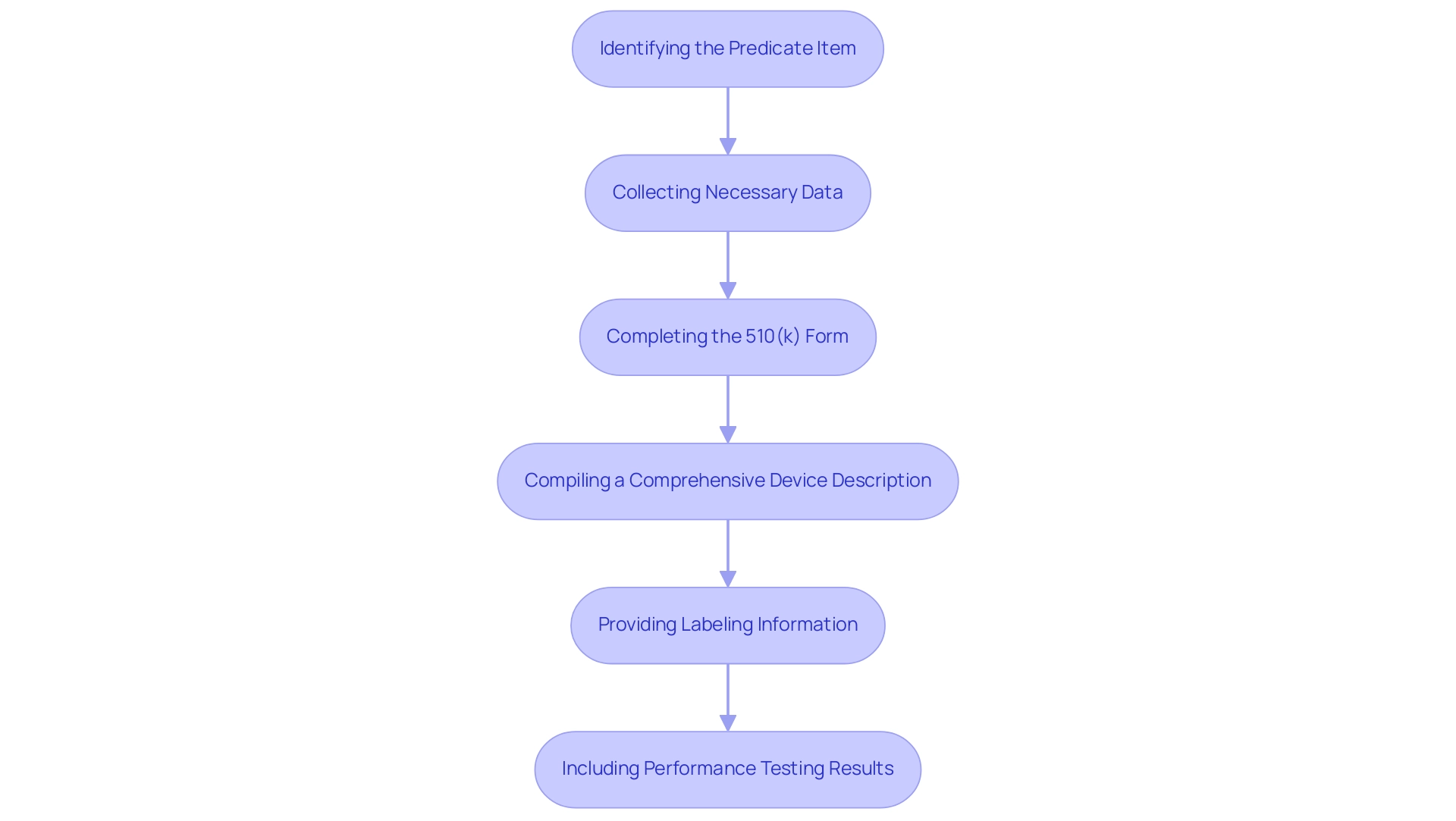
Preparing a 510(k) submission necessitates a systematic approach, which includes understanding the 510k meaning and encompasses several critical steps:
-
Identifying the Predicate Item:
Begin by researching similar medical products online and utilizing the FDA's 510(k) database to ensure substantial equivalence. This process is essential, as the Executive Summary must describe your medical product in comparison to the predicate product and summarize all of the testing you've conducted.
-
Collecting Necessary Data:
Gather comprehensive data that establishes the safety and efficacy of the apparatus.
Be prepared to address common challenges in the application process, such as a lack of understanding of the requirements and insufficient documentation.
-
Completing the 510(k) Form:
Accurately fill out the required forms, ensuring all information is complete.
-
Compiling a Comprehensive Device Description:
Provide a detailed description of the device, outlining its intended use and technological characteristics.
-
Providing Labeling Information:
Include all relevant labeling materials that meet FDA requirements.
-
Including Performance Testing Results:
Present any performance testing data that supports your claims. Participating in a pre-submission meeting with the FDA is strongly advised, as it provides clarification of any inquiries and guidance on the application process.
Furthermore, maintaining thorough documentation and adhering to the latest FDA guidelines are essential for a successful application. As noted by the FDA, manufacturers may submit changes as a Special 510(k) when they determine that no additional verification or validation testing is necessary, provided there is a clear rationale for that conclusion. This highlights the significance of grasping the 510k meaning and the nuances of the application process to avoid common pitfalls.
For example, firms often begin by searching for similar medical products online to identify potential predicate items, which helps ensure that the chosen predicate meets substantial equivalence criteria. Ana Criado, our Director of Regulatory Affairs, brings extensive experience from her leadership roles at INVIMA and as a consultant for global companies like General Electric and Omron Healthcare, alongside her academic involvement as a biomedical engineering professor. Her expertise in health economics and Regulatory Affairs, particularly in the context of cannabis regulation through her role as CEO of Mahu Pharma, positions her to provide invaluable insights into navigating complex regulatory landscapes.
Specifically, Ana can assist in identifying the appropriate predicate products and addressing documentation challenges, leveraging her deep understanding of regulatory requirements to help firms avoid common filing pitfalls.
Navigating the FDA Review Process for 510(k) Submissions
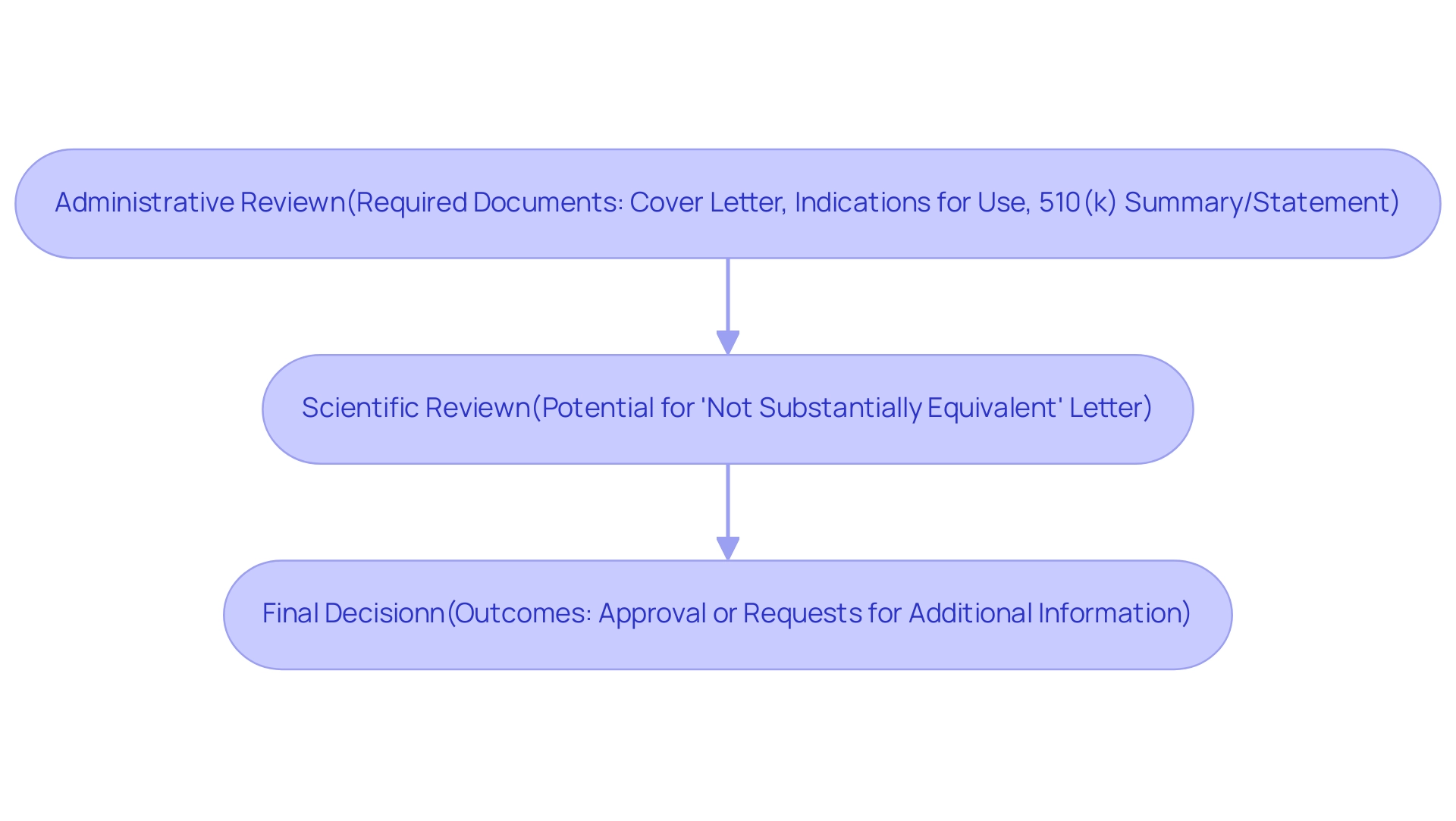
Upon submitting a 510(k), the FDA generally conducts its review within an average of 120 days, which is important for understanding the 510k meaning and reflects the agency's commitment to efficient processing. The review process comprises several distinct stages:
- Administrative review
- Scientific review
- Final decision
Initially, during the administrative review, the FDA verifies the completeness of the application, which must include key summary documents such as a cover letter, indications for use, and either a 510(k) summary or statement.
Following this, the scientific review phase critically evaluates the data to ascertain whether the apparatus is substantially equivalent to its predicate apparatus. If the proposal does not meet the necessary criteria, the FDA may issue a 'not substantially equivalent' letter, requiring further clarification or additional data from the submitter.
Significantly, specialists such as Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, who possesses over five years of experience at INVIMA managing regulatory compliance, and Katherine Ruiz, an authority in regulatory matters for medical products and in vitro diagnostics in Colombia with considerable expertise in health regulations, stress the significance of diligence and thoroughness in preparing applications, highlighting the 510k meaning.
As Dr. Hugh Harvey noted, the slowest review time recorded was 287 days for BrainLab's BOLD MRI Mapping tool, illustrating the variability in the review timeline. Professionals must be prepared for potential requests for additional information, as there is a response window of 180 days before a submission may be withdrawn from the process.
Furthermore, a recent survey of industry leaders revealed that nearly 90 percent now prioritize US regulatory approval over the EU due to challenges with the new MDR, underscoring the shifting landscape of medical product approvals.
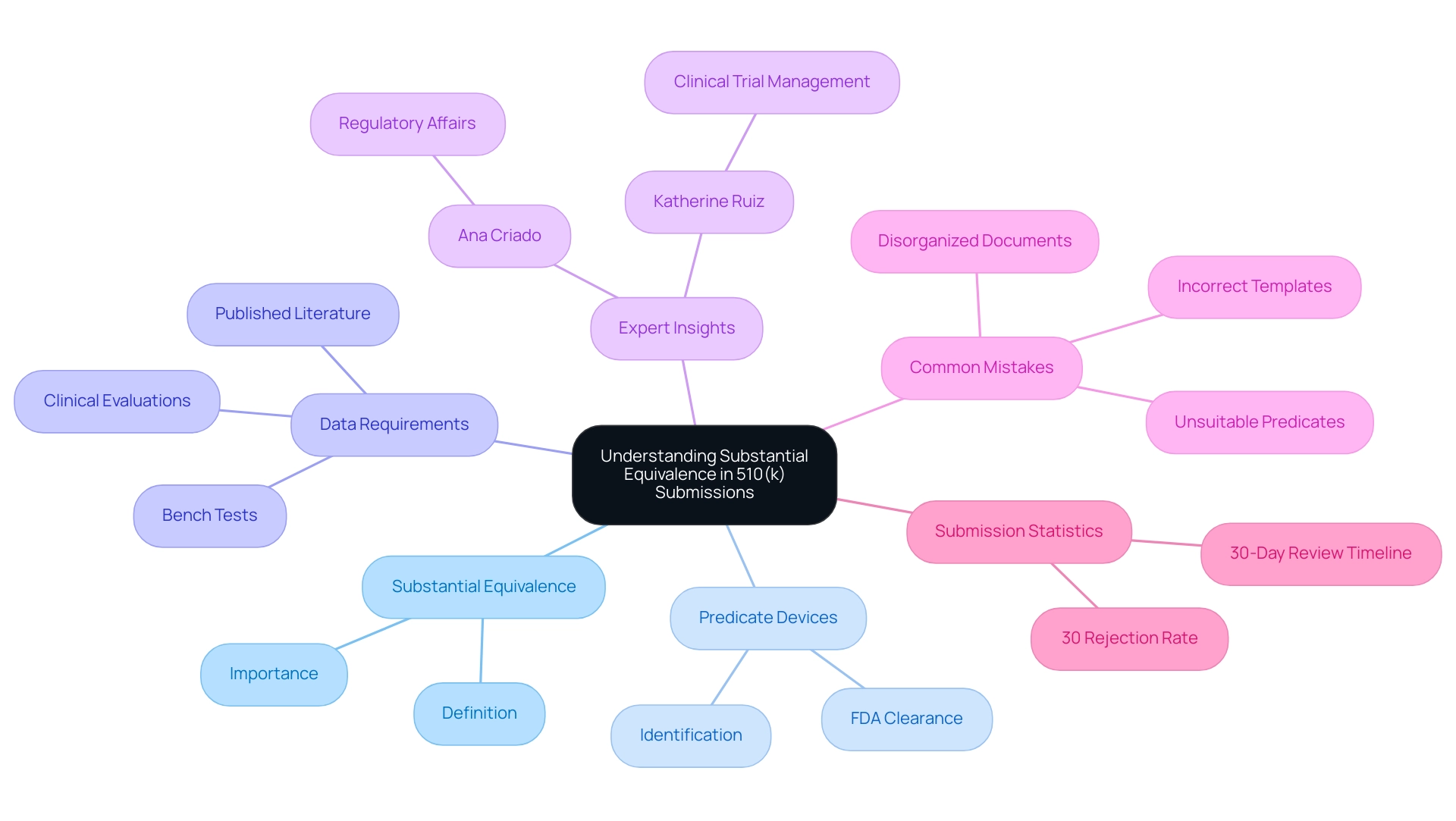
Understanding Substantial Equivalence in 510(k) Submissions
Substantial equivalence indicates that a new medical instrument is as safe and effective as an already legally marketed predicate instrument. To substantiate this claim, manufacturers must present comparative data that illustrates similarities in design, materials, intended use, and performance characteristics. Acceptable forms of this data may include results from bench tests, clinical evaluations, and relevant published literature.
Identifying a predicate device with existing FDA clearance is essential, as it establishes the framework for comparison. According to the FDA, 'In cases where manufacturers conclude under their design control procedures that no additional verification or validation testing is necessary to assess a change that otherwise requires clearance of a 510(k), the 510k meaning allows manufacturers to present these changes as a Special 510(k) with a clear rationale supporting their conclusion that no performance data are necessary.' As emphasized in 2022, around 30% of 510(k) applications encountered rejection during initial review, which underscores the critical importance of meticulously documenting and justifying claims of substantial equivalence to streamline FDA evaluation and enhance the likelihood of successful approval, illustrating the 510k meaning.
By strategically separating intended use from technological characteristics and thoroughly analyzing product codes, as outlined in the case study titled 'Strategies for Successful 510(k) Applications,' manufacturers can significantly improve their outcomes. Furthermore, experts like Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, and Katherine Ruiz, an expert in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, provide invaluable insights into navigating the complexities of regulatory compliance and clinical trial management. Their expertise encompasses comprehensive clinical trial management services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
This ensures that submission processes align with both local and international standards.
Conclusion
The complexities of the 510(k) submission process are pivotal for medical device manufacturers aiming to navigate the regulatory landscape effectively. As detailed, the 510(k) serves as a vital premarket notification that allows manufacturers to demonstrate substantial equivalence to existing devices, facilitating a quicker and more cost-effective route to market for medium-risk devices. With approximately 10,000 submissions received annually and an impressive approval rate of around 90%, understanding the eligibility criteria, preparation steps, and the FDA review process is essential for success in this arena.
Key steps in preparing a 510(k) submission include:
- Identifying suitable predicate devices
- Compiling comprehensive data
- Ensuring meticulous documentation
Engaging in pre-submission meetings with the FDA can further clarify the process and enhance the likelihood of approval. The emphasis on substantial equivalence underscores the need for robust comparative data, as approximately 30% of submissions faced rejection in 2022 due to inadequate justification of this critical criterion.
As advancements in artificial intelligence and machine learning technologies reshape the medical device landscape, the importance of staying informed about regulatory practices cannot be overstated. Insights from experts in the field highlight the need for diligence and thoroughness, ensuring compliance with evolving standards while fostering innovation. In this rapidly changing environment, a solid grasp of the 510(k) submission process will empower manufacturers to bring safer and more effective medical devices to market, ultimately benefiting patients and the healthcare system at large.
Frequently Asked Questions
What is the purpose of the 510(k) submission?
The 510(k) submission serves as a premarket notification to the U.S. Food and Drug Administration (FDA), allowing medical product manufacturers to demonstrate that their product is safe and effective based on substantial equivalence to an existing legally marketed item, known as a predicate item.
Which classes of medical products require a 510(k) submission?
The 510(k) submission is particularly important for Class II and III products. Class II products typically must submit a 510(k) to show substantial equivalence, while Class III products usually require Premarket Approval (PMA) instead.
What are the classifications of medical devices based on risk levels?
Medical devices are categorized into three primary groups based on their risk level: Class I (low risk), Class II (moderate risk), and Class III (high risk).
What is the approval rate for 510(k) applications?
The FDA receives approximately 10,000 510(k) applications annually, with an impressive approval rate of around 90%.
How does the 510(k) process differ from the Premarket Approval (PMA) process?
The 510(k) process is more streamlined and allows manufacturers to obtain market clearance without the extensive preclinical and clinical testing required for a PMA, making it a quicker and more economical route for medium-risk products.
What recent trends have been observed in the 510(k) process?
There has been a significant increase in the number of AI and machine learning-enabled products cleared through the 510(k) process, with more such products cleared via this pathway than through De Novo classification.
What role does real-world evidence (RWE) play in the 510(k) process?
Real-world evidence generated through initiatives like the National Evaluation System for health Technologies (NEST) may be used for post-market surveillance and to support premarket regulatory decision-making, as well as for expanded indications for use after clearance or approval.
Why is understanding the 510(k) process important for clinical research professionals?
Understanding the 510(k) process is crucial for clinical research professionals to avoid regulatory missteps and ensure compliance with essential standards, especially in the context of the growing prevalence of innovative medical technologies.

