Introduction
In the realm of medical device manufacturing, the importance of accelerated shelf life testing cannot be overstated. This innovative methodology not only facilitates the assessment of product longevity and stability but also accelerates the path to market for critical healthcare solutions.
By simulating the aging process under controlled environmental conditions, manufacturers can glean vital insights into the performance and safety of their devices, ensuring compliance with stringent regulatory standards such as ISO 11607 and ASTM F1980.
As the industry increasingly prioritizes rapid innovation and patient safety, understanding the nuances of accelerated shelf life testing becomes essential for stakeholders aiming to enhance product reliability and meet evolving market demands.
Through a combination of scientific rigor and practical application, this testing approach stands as a cornerstone in the pursuit of high-quality medical devices.
Introduction to Accelerated Shelf Life Testing for Medical Devices
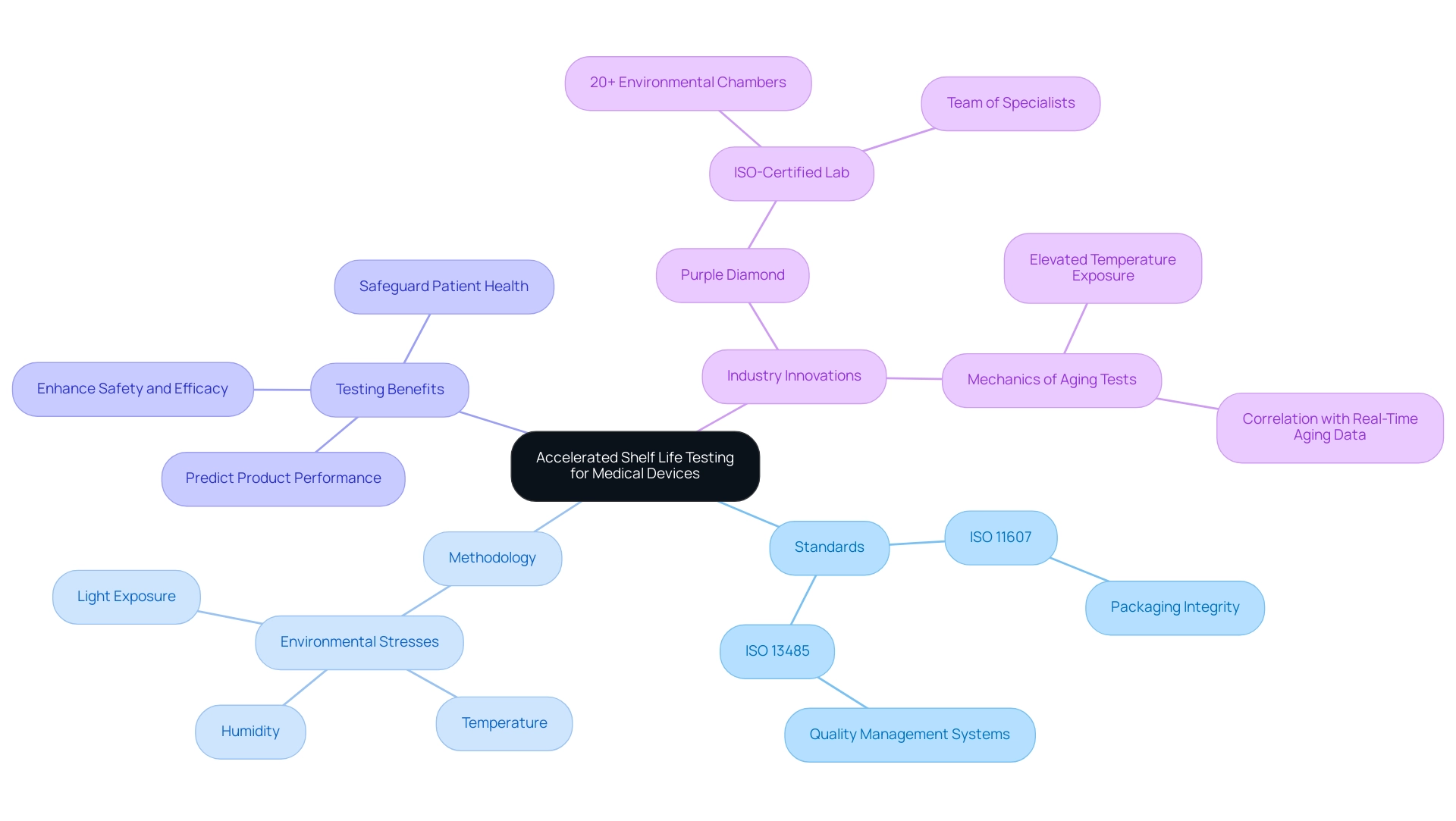
The methodology of accelerated shelf life testing medical device plays a crucial role in evaluating the longevity and stability of medical equipment by simulating the aging process in a shortened timeframe. As per ISO 11607, compliance with these evaluation protocols is crucial for packaging integrity, while ISO 13485 specifies the quality management systems required for medical product production. This testing process for the accelerated shelf life testing medical device is not simply a regulatory requirement; it is essential for manufacturers aiming to predict product performance over its intended shelf life without the lengthy wait for actual aging data.
By exposing equipment to various environmental stresses—including temperature fluctuations, humidity variations, and exposure to light—researchers can effectively assess the durability and functionality of these items. As Karl J. Hemmerich, general manager at Isomedix Corp., articulates,
Applying accelerated-aging test techniques in conjunction with a comprehensive knowledge of the materials involved is a prudent method of doing business, with the benefits of early introduction far outweighing the minimal risk of premature failure.
This approach not only enhances safety and efficacy throughout the device lifecycle but also safeguards patient health by ensuring that medical devices meet stringent performance standards.
Additionally, real-world examples, such as the case study titled 'Mechanics of Aging Tests,' demonstrate how rapid aging tests expose products to elevated temperatures to study their aging reactions, allowing for quicker data collection. These results must be correlated with Real-Time Aging data to be considered valid by regulatory authorities. Furthermore, companies such as Purple Diamond are progressing the field by providing accelerated shelf life testing medical device evaluations in an ISO-certified lab, equipped with over 20 environmental chambers and a team of specialists, thereby highlighting current industry practices and innovations.
Key Methodologies and Standards: ASTM F1980 and Beyond
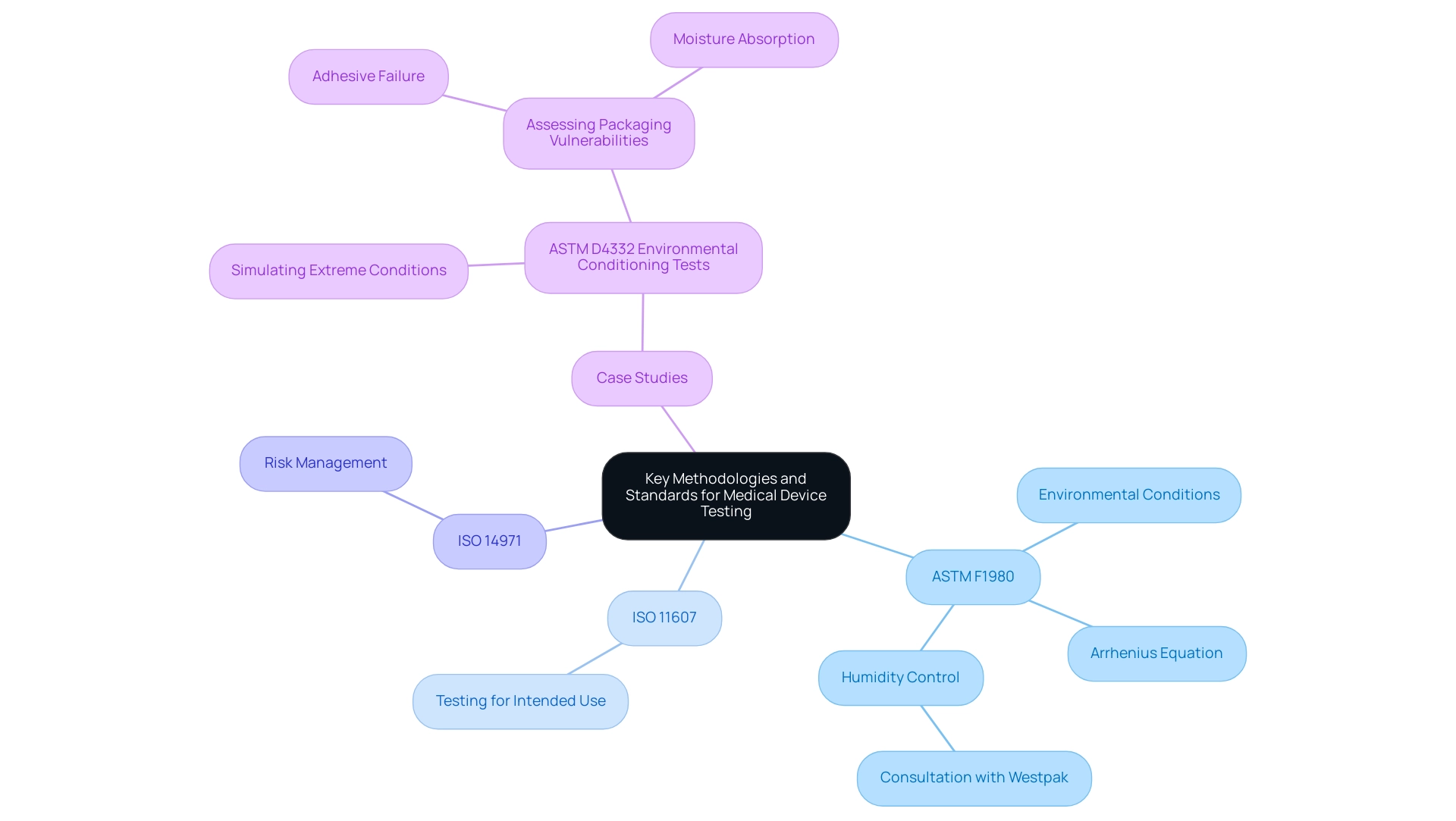
The ASTM F1980 standard functions as a thorough guideline for conducting accelerated shelf life testing medical device assessments, which are essential for confirming the durability and safety of medical instruments. It delineates specific environmental conditions, including temperature and humidity, under which tests must be conducted, along with stipulations regarding the duration and frequency of these assessments. The Arrhenius Equation is especially significant in this context, as it aids in forecasting the effects of temperature on the deterioration rates of medical instruments, thus offering a scientific foundation for aging tests.
Complementing ASTM F1980, the standards ISO 11607 and ISO 14971 are pivotal in ensuring that medical equipment undergoes rigorous testing pertinent to its intended use and safety. Notably, Westpak emphasizes that if humidity during accelerated aging is to be controlled, it is essential to confer with their sales team to determine the appropriate relative humidity (RH) level to be specified in the test plan. Furthermore, case studies such as the ASTM D4332 Environmental Conditioning Tests illustrate the real-world application of these standards, simulating extreme environmental conditions to assess packaging vulnerabilities like adhesive failure or moisture absorption during transit.
By following these established standards, organizations can improve reliability and ensure compliance with regulatory requirements, ultimately contributing to the safety and effectiveness of medical equipment in clinical settings, particularly through accelerated shelf life testing medical device.
Benefits of Accelerated Shelf Life Testing in Medical Device Safety
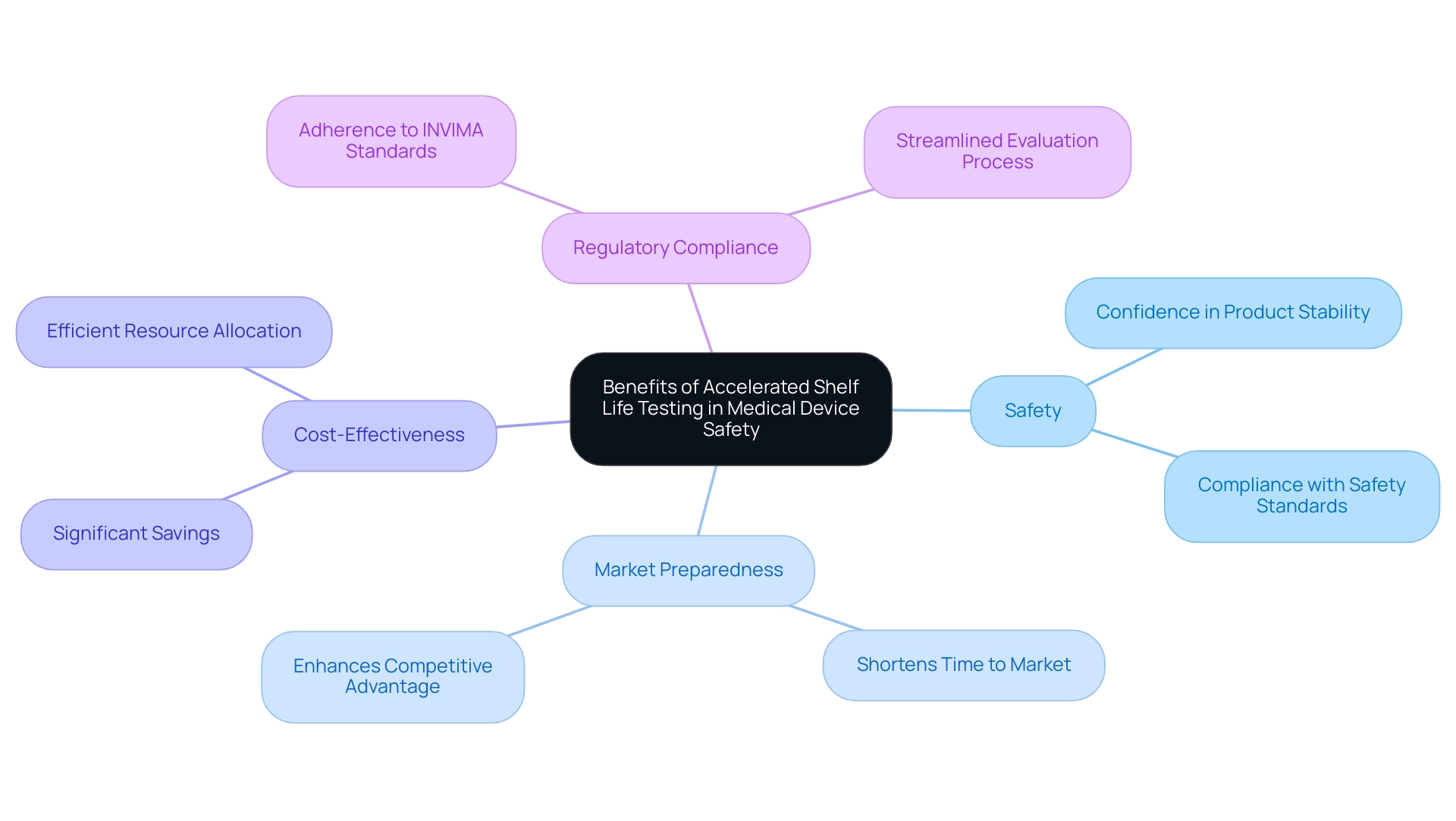
The accelerated shelf life testing medical device plays a vital role in the medical equipment sector, offering numerous advantages that directly affect safety and market preparedness. Bioaccess®, with over 20 years of expertise in managing medical equipment clinical studies, including Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, provides a robust foundation for manufacturers seeking to enhance credibility. By utilizing accelerated shelf life testing medical device techniques, producers can substantially shorten the time to market for new products, thereby enhancing their competitive advantage.
This strategy not only fosters greater confidence in product stability but also reinforces risk management approaches, ensuring items comply with safety standards prior to entering the market. As remarked by Keith E. Belk, following regulatory standards is crucial, especially considering the supervision from INVIMA, Colombia's National Food and Drug Surveillance Institute, categorized as a Level 4 health authority by PAHO/WHO.
Furthermore, the use of accelerated shelf life testing medical device has been demonstrated to be more economical than conventional methods. While traditional assessment can be time-consuming and costly, utilizing accelerated shelf life testing medical device approaches can result in significant savings for manufacturers while ensuring that patients obtain dependable and safe products. This cost-effectiveness is a critical advantage that allows manufacturers to allocate resources more efficiently.
Recent reports quantify the overall acceptability of products undergoing this evaluation at 7.76 ± 0.07, underscoring the method's effectiveness in ensuring product quality. A notable case study titled 'Shelf Life Estimation Using Kinetic Parameters' demonstrated that analyzing temperature dependence in water-jacketed confections (WJCs) using the Arrhenius equation allowed researchers to determine a targeted shelf life of six months. This example demonstrates that through improved shelf life assessments, manufacturers can optimize their development processes while enhancing the safety and effectiveness of their medical products as they prepare for 2024 and beyond.
Moreover, bioaccess® provides a personalized strategy for overseeing clinical trials, adjusting services to fulfill the distinct requirements of each client, which further improves the overall efficiency of the expedited evaluation process.
Comparing Accelerated Aging and Real-Time Aging Testing
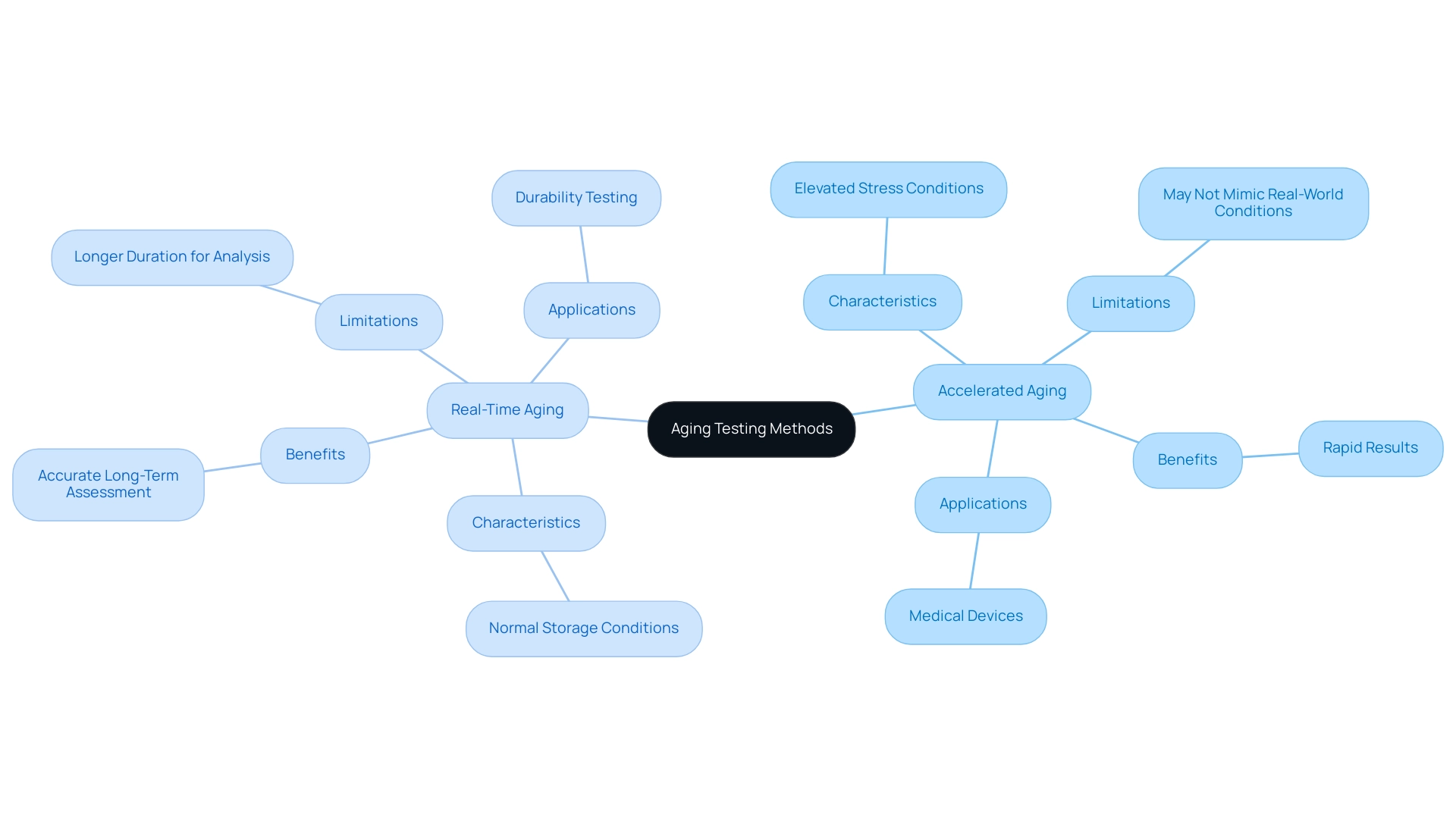
Accelerated shelf life testing medical devices simulates long-term aging by subjecting them to elevated stress conditions, while real-time aging assessments examine the effects of environmental elements over extended periods under normal storage conditions. The main difference lies in the schedule of outcomes: rapid evaluation can produce results considerably quicker, but it may not reliably mimic real-world circumstances. In contrast, real-time aging evaluation offers a more accurate assessment of performance over time, albeit requiring a longer duration for analysis.
As noted by Florian Sutter from the German Aerospace Center (DLR), the degradation of glass and aluminum mirrors is analyzed under accelerated shelf life testing medical device conditions and compared to outdoor exposure results, emphasizing the importance of correlating accelerated aging data with real-time aging outcomes. For instance, 8 samples have been previously cycled 100 times according to IEC 61215, Test 10.11, illustrating the rigorous evaluation conditions that can reveal insights into durability. The case study titled 'Mechanics of Accelerated Shelf Life Testing Medical Device' further supports this, demonstrating how elevated temperatures affect aging reactions in various environments.
Furthermore, Westpac will create a comprehensive report outlining the aging conditions, assessment standards, and equipment used, emphasizing ongoing developments in the field. Comprehending these distinctions is essential for manufacturers, as it allows them to choose the most appropriate evaluation method based on specific item specifications and regulatory expectations. Future research seeks to enhance evaluation techniques by contrasting rapid aging data with findings from outdoor exposure, ensuring that the insights obtained from both approaches contribute to more precise assessments.
Case Studies: Real-World Applications of Accelerated Shelf Life Testing
A persuasive case study highlights a manufacturer of orthopedic implants that utilized expedited shelf life evaluation to confirm the durability of their items under simulated extreme conditions. By applying environmental stresses that replicated years of usage, the company was able to pinpoint potential failure points and make critical design modifications prior to the launch, thus enhancing overall safety and reliability.
In another case, a firm that encountered regulatory examination due to item failures effectively carried out enhanced shelf life evaluations to show adherence to established safety standards. This proactive approach not only addressed the immediate concerns but also played a vital role in regaining market confidence. According to a recent study, overall acceptability scored at 5.80 ± 0.01, highlighting the effectiveness of expedited shelf life evaluation in ensuring product quality.
Furthermore, the case study titled 'Assessing Drug Product Shelf Life Using the Accelerated Stability Assessment Program,' which focuses on the GLPG4399 capsule formulation, provides additional insights into stability assessment. Kevin Roeleveld points out that this program emphasizes the significance of expedited evaluations in upholding safety standards.
These examples highlight the practical benefits of accelerated shelf life testing medical devices, illustrating its crucial role in ensuring the safety and effectiveness of medical devices while simultaneously reinforcing industry standards. With over 20 years of experience in Medtech, bioaccess® has the expertise to effectively manage accelerated shelf life testing medical device within its Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies.
Coupled with the regulatory oversight provided by INVIMA as a Level 4 health authority by PAHO/WHO, this approach positions companies to navigate complex clinical landscapes more effectively.

Conclusion
Accelerated shelf life testing is an invaluable approach in the medical device industry, serving as both a regulatory requirement and a strategic advantage for manufacturers. By simulating the aging process through controlled environmental conditions, this methodology not only assesses product longevity and stability but also accelerates the introduction of innovative healthcare solutions to the market. Compliance with standards such as ISO 11607 and ASTM F1980 ensures that devices meet stringent safety and performance criteria, ultimately safeguarding patient health.
The benefits of accelerated shelf life testing extend beyond mere compliance; they enhance product reliability, streamline development processes, and offer significant cost savings. By effectively predicting device performance over time, manufacturers can mitigate risks and bolster market confidence. Real-world case studies further illustrate the practical applications of this testing approach, highlighting how it enables companies to identify potential failure points and implement design improvements before product launch.
In conclusion, as the medical device landscape continues to evolve, the importance of accelerated shelf life testing will only grow. Stakeholders must embrace this methodology to enhance product safety and efficacy, ensuring that medical devices meet the increasing demands of healthcare providers and patients alike. By prioritizing accelerated testing, manufacturers position themselves at the forefront of innovation while contributing to a safer and more reliable healthcare ecosystem.
Frequently Asked Questions
What is the purpose of accelerated shelf life testing for medical devices?
Accelerated shelf life testing simulates the aging process of medical equipment in a shortened timeframe to evaluate longevity and stability, helping manufacturers predict product performance over its intended shelf life.
Which standards govern accelerated shelf life testing for medical devices?
The primary standards include ISO 11607, which focuses on packaging integrity, and ISO 13485, which specifies the quality management systems required for medical product production. ASTM F1980 also provides guidelines for conducting these assessments.
Why is compliance with these testing protocols important?
Compliance is crucial for ensuring packaging integrity, confirming the durability and safety of medical instruments, and meeting regulatory requirements, ultimately contributing to the safety and effectiveness of medical equipment.
How does accelerated shelf life testing assess medical device durability?
The testing exposes equipment to various environmental stresses, such as temperature fluctuations, humidity variations, and light exposure, allowing researchers to assess the durability and functionality of the devices.
What is the significance of the Arrhenius Equation in accelerated shelf life testing?
The Arrhenius Equation helps forecast the effects of temperature on the deterioration rates of medical instruments, providing a scientific basis for aging tests.
Can you give an example of how accelerated aging tests are applied?
A case study titled 'Mechanics of Aging Tests' demonstrates that rapid aging tests expose products to elevated temperatures to study their aging reactions, facilitating quicker data collection that must be correlated with real-time aging data for regulatory validation.
What role do companies like Purple Diamond play in accelerated shelf life testing?
Companies like Purple Diamond offer accelerated shelf life testing evaluations in ISO-certified labs, equipped with environmental chambers and specialists, highlighting advancements and current practices in the industry.
What are the implications of not controlling humidity during accelerated aging tests?
If humidity is not controlled, it can affect the test results, making it essential to consult with experts to determine the appropriate relative humidity levels to include in the test plan.
How do established standards contribute to the testing process?
Following established standards like ASTM F1980, ISO 11607, and ISO 14971 improves reliability, ensures compliance with regulatory requirements, and ultimately enhances the safety and effectiveness of medical equipment in clinical settings.

