Introduction
The development of medical devices is a complex process that necessitates adherence to rigorous standards, with ASTM standards at the forefront of ensuring quality, safety, and efficacy. These internationally recognized benchmarks serve as a foundation for manufacturers, guiding them through the intricate landscape of regulatory compliance and industry best practices.
As the medical technology field evolves, the significance of these standards becomes increasingly apparent, not only in mitigating risks associated with device production but also in enhancing patient outcomes and fostering trust within the healthcare community.
This article delves into the critical role of ASTM standards in medical device development, exploring:
- Key standards
- Their implementation in clinical trials
- The challenges manufacturers face
- The future trajectory of these essential guidelines in an era marked by rapid technological advancements.
The Importance of ASTM Standards in Medical Device Development
At bioaccess™, we understand that industry guidelines are crucial to the creation of healthcare instruments, setting vital benchmarks for quality, safety, and effectiveness. These globally acknowledged criteria, established by ASTM International, guide manufacturers in ensuring compliance with regulatory requirements and adherence to ASTM standards for medical devices. By adhering to these guidelines, companies not only reduce risks linked to medical devices but also improve patient outcomes and promote trust among healthcare professionals and patients.
Our dedication to innovation and quality in healthcare motivates us to participate in this cooperative approach, as emphasized by scientists from the Cambridge Polymer Group, who state,
Our involvement highlights the cooperative nature of guidelines development, bringing together expertise from industry, academia, and regulatory bodies.
Moreover, adherence to industry guidelines streamlines the regulatory approval process and reduces the risk of product recalls, which is crucial for upholding the integrity of the healthcare system. This is particularly relevant as we advance comprehensive clinical trial management services, including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting.
The impact of these medtech clinical studies on local economies is profound, contributing to job creation, economic growth, healthcare improvement, and international collaboration. A practical example of industry guidelines in action can be observed in the case study on suggested humidity levels for accelerated aging, where regulated humidity is recommended based on material sensitivity, with documentation necessary for any uncontrolled conditions according to F1980-21. Additionally, Westpak testing labs are ISTA certified to perform a variety of tests, underscoring the credibility of compliance efforts within the industry.
As the industry progresses, the significance of ASTM standards for medical devices remains crucial, especially as we anticipate 2024 and the future. Grasping these guidelines allows producers to balance adherence with material performance, ensuring that products are not only safe but also effective in their intended application. We welcome you to become a member of our committed team at bioaccess™ and participate in the revolution in healthcare technologies.
Together, we can advance healthcare through innovation and quality. APPLY TODAY!
Key ASTM Standards and Testing Methods for Medical Devices
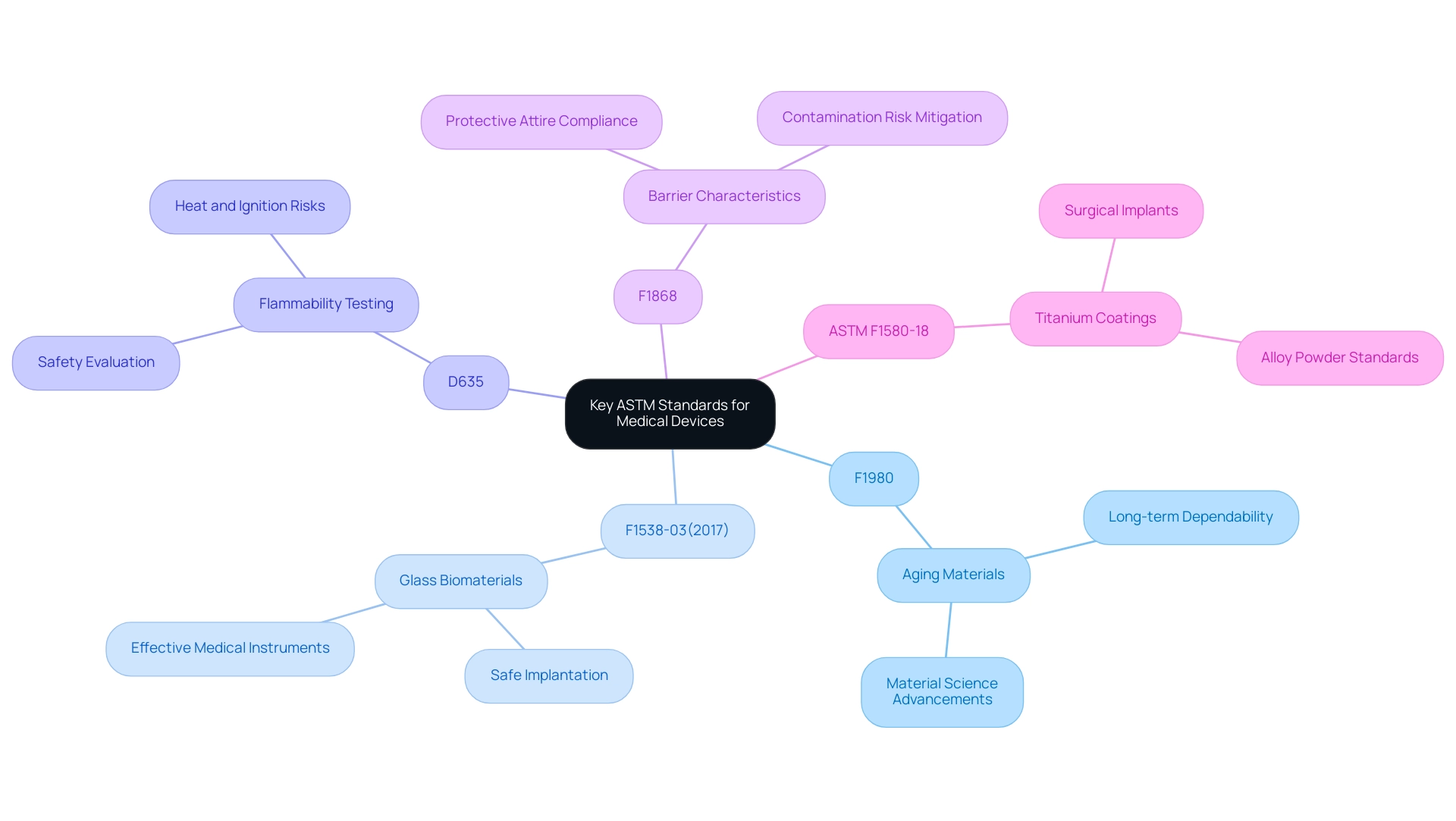
A thorough comprehension of relevant regulations is crucial for the efficient assessment of healthcare products, as these guidelines encompass 24 classifications of healthcare items, including surgical tools and automated analysis. Key criteria include:
- F1980: This guideline outlines principles for aging materials utilized in healthcare equipment, ensuring their long-term dependability and performance. Its significance is emphasized by ongoing advancements in material science, which aim to enhance the durability of healthcare implants.
- F1538-03(2017): This guideline specifies glass and glass ceramic biomaterials for implantation, emphasizing its essential role in the creation of safe and effective medical instruments.
- D635: This guideline outlines testing methods for the flammability of plastic materials, a crucial factor in evaluating the safety of equipment. The consequences of flammability testing are especially significant in equipment that may face heat or ignition sources, making compliance with this guideline essential.
- F1868: This guideline emphasizes assessing the barrier characteristics of protective attire, which is especially pertinent for devices that engage directly with patients. Ensuring that protective gear complies with these criteria mitigates risks associated with contamination and infection.
- ASTM F1580-18: This guideline establishes protocols for titanium and titanium-6 aluminum-4 vanadium alloy powders for coatings of surgical implants, providing a real-world example of how these criteria are applied in practice.
By comprehending and applying these guidelines, producers can guarantee that their healthcare products not only fulfill regulatory obligations but also emphasize safety and effectiveness for users. Frequent consultation with these guidelines throughout the design and testing stages is essential for attaining thorough compliance and maintaining quality assurance in healthcare production. As stated by researchers from the Cambridge Polymer Group, our participation highlights the cooperative essence of guidelines development, uniting knowledge from industry, academia, and regulatory entities. This partnership is essential for enhancing the safety and dependability of healthcare instruments, in accordance with the most recent guidelines and testing procedures.
Implementing ASTM Standards in Clinical Trials
Incorporating industry guidelines into clinical trials requires careful planning and protocol creation. Researchers must first recognize the particular criteria relevant to their healthcare apparatus and trial framework. For instance, F2503 outlines critical guidelines for labeling medical devices, ensuring that they convey essential information regarding safety and effectiveness.
In the same way, F2475 emphasizes the testing methods for surgical instruments, which is crucial for upholding high safety criteria. According to a study by the Consumer Product Safety Commission (CPSC), adherence to industry guidelines has led to a noteworthy reduction in product-related injuries over the past decade. Following these guidelines not only protects participant safety but also enhances the credibility of the trial results.
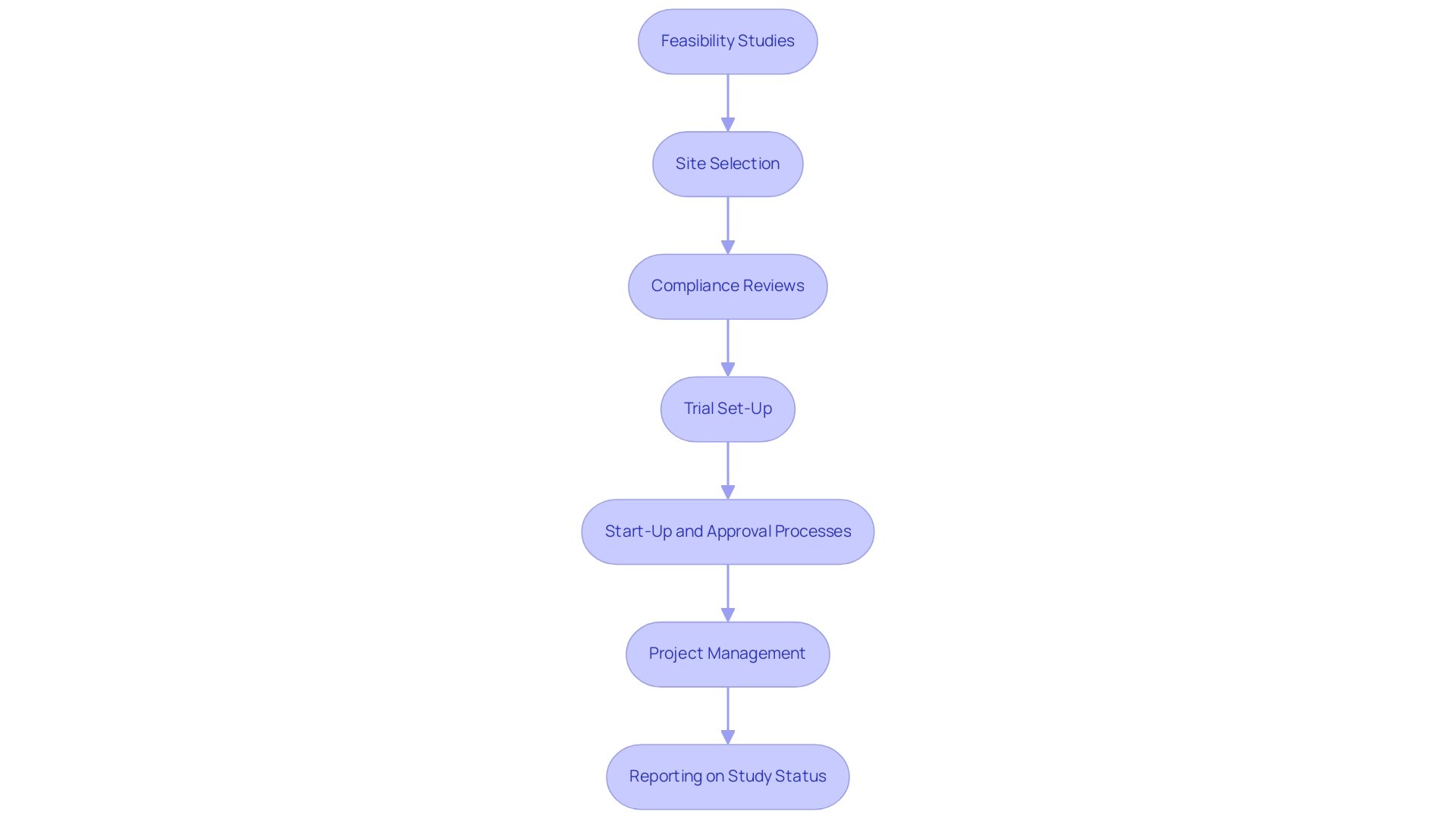
A comprehensive approach involves:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial set-up
- Start-up and approval processes
- Project management
- Reporting on study status, inventory, and serious and non-serious adverse events
A relevant case study, titled 'Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements to Determine the Corrosion Susceptibility of Small Implant Devices,' outlines a method for assessing corrosion susceptibility, providing critical data for evaluating the longevity and safety of small implants. Throughout the clinical trial, it is essential to perform regular audits and compliance checks to ensure ongoing adherence to industry guidelines.
This proactive approach mitigates risks and preserves data integrity, ultimately enhancing the reliability of the study outcomes. Recent advancements in testing protocols have further highlighted their significance in clinical trials, emphasizing the necessity for adherence to attain optimal outcomes. Moreover, the data indicating that 29 percent of participants thought ethnic group should NOT be part of their EHR system emphasizes wider implications of guidelines and adherence in clinical research, considering the difficulties startups in the healthcare field encounter in maneuvering through regulatory environments and recruitment challenges.
Challenges in Adhering to ASTM Standards
Following industry guidelines presents a complex array of challenges for medical device producers, greatly affecting their operational efficiency and product development schedules. The complexity of these regulations requires a commitment to ongoing education, as staying updated with changing guidelines is essential. Regular updates and revisions to industry guidelines often disrupt development processes and can result in expensive delays.
Smaller manufacturers, in particular, often face hurdles due to limited financial and human resources, making it difficult to establish and maintain effective compliance programs. As highlighted by industry expert Katherine Ruiz, who specializes in regulatory matters for health products and in vitro diagnostics in Colombia, stringent quality controls in manufacturing are essential for safeguarding consumer health and safety, which corresponds with the significance of rigorous compliance across all sectors. To mitigate these challenges, stakeholders should prioritize investing in training and development to enhance staff expertise.
Establishing robust compliance management systems and fostering a culture of quality and safety are essential strategies. Notably, our comprehensive clinical trial management services encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Detailed reporting on study status, inventory, and both serious and non-serious adverse events
These services provide centralized management solutions that effectively address compliance and operational challenges.
Working together with regulatory advisors and industry specialists can offer essential insights into managing compliance efficiently, assisting manufacturers not only in meeting industry benchmarks but also in maintaining the trust and safety vital to their market position. Furthermore, adherence to international conventions has been shown to reduce workplace incidents by up to 30%, further emphasizing the significance of a proactive approach to compliance.
The Future of ASTM Standards in Medical Devices
As the medical equipment industry progresses with technological advancements, the ASTM standards for medical devices are set to experience substantial changes to maintain safety, efficacy, and innovation. Key emerging trends, such as the increase of personalized medicine and the incorporation of artificial intelligence into equipment functionality, are anticipated to spur the creation of new criteria or the modification of current ones. Moreover, with a growing focus on cybersecurity, the establishment of strong protocols in this area will be essential to protect patient information and equipment integrity.
Significantly, the international voting members must attain a minimum of 90% positive vote with at least 60% involvement from the voting committee, emphasizing the cooperative essence of guideline development. As worldwide markets grow, relevant guidelines are expected to converge more closely with international regulations, enabling easier market access for healthcare products across borders. Stakeholders are encouraged to maintain active involvement with relevant organizations to stay informed about these developments and to contribute to the formulation of guidelines that adhere to ASTM standards for medical devices, which will shape the future landscape of medical equipment safety and effectiveness.
As Stephen Spiegelberg remarked, 'The development of guidelines is essential for promoting innovation and guaranteeing the safety of new technologies.' Recent guidelines such as F2051-00(2022) for implantable saline-filled breast prostheses exemplify the forward momentum in unification efforts, while the F2528-06(2023) for enteral feeding tools with retention balloons illustrates the ongoing refinement of testing methods to meet contemporary needs. Additionally, key market players such as SGS SA, Eurofins Scientific, Intertek Group, and TÜV SÜD play a vital role in shaping the competitive landscape and driving innovation within the medical device testing services market, further influencing the development and compliance of ASTM standards for medical devices.
Conclusion
The importance of ASTM standards in medical device development cannot be overstated. These standards provide a crucial framework that ensures quality, safety, and efficacy throughout the manufacturing process. By adhering to ASTM guidelines, manufacturers can navigate the complexities of regulatory compliance while enhancing patient outcomes and building trust within the healthcare community.
Key ASTM standards, such as:
- F1980 for aging materials
- F1538 for biomaterials
play a vital role in the evaluation and testing of medical devices. Their implementation in clinical trials not only safeguards participant safety but also enhances the credibility of the results, paving the way for innovations that align with the latest safety protocols. However, manufacturers face significant challenges in maintaining compliance, particularly smaller entities that may struggle with limited resources. Continuous education and robust compliance management systems are essential to overcome these hurdles and ensure adherence to evolving standards.
Looking forward, the evolution of ASTM standards will be critical in addressing emerging trends such as personalized medicine and cybersecurity. As the landscape of medical technology continues to shift, active engagement with ASTM International will empower stakeholders to contribute to the development of standards that not only enhance safety and effectiveness but also foster innovation. The future of medical devices is bright, with ASTM standards serving as a cornerstone of quality and reliability in this dynamic industry.
Frequently Asked Questions
Why are industry guidelines important in healthcare instrument creation?
Industry guidelines are crucial as they set benchmarks for quality, safety, and effectiveness, helping manufacturers ensure compliance with regulatory requirements and ASTM standards for medical devices.
How do industry guidelines benefit medical device manufacturers?
By adhering to industry guidelines, manufacturers reduce risks associated with medical devices, improve patient outcomes, and foster trust among healthcare professionals and patients.
What role does bioaccess™ play in the development of industry guidelines?
Bioaccess™ participates in the cooperative approach to guidelines development, collaborating with industry, academia, and regulatory bodies to enhance healthcare innovations and quality.
How do industry guidelines affect the regulatory approval process?
Adherence to industry guidelines streamlines the regulatory approval process and minimizes the risk of product recalls, which is vital for maintaining the integrity of the healthcare system.
What impact do medtech clinical studies have on local economies?
Medtech clinical studies contribute to job creation, economic growth, healthcare improvement, and international collaboration.
Can you provide an example of industry guidelines in action?
An example is the case study on humidity levels for accelerated aging, which recommends regulated humidity based on material sensitivity and requires documentation for any uncontrolled conditions according to F1980-21.
What are some key ASTM guidelines relevant to healthcare products?
Key guidelines include: F1980 (principles for aging materials in healthcare equipment), F1538-03(2017) (specifications for glass and glass ceramic biomaterials for implantation), D635 (testing methods for the flammability of plastic materials), F1868 (assessment of barrier characteristics of protective attire), and ASTM F1580-18 (protocols for titanium alloys for surgical implants).
How do producers ensure compliance with industry guidelines?
Producers must frequently consult and apply these guidelines throughout the design and testing stages to ensure compliance and maintain quality assurance in healthcare production.




