Introduction
The realm of medical devices is intricately tied to the concept of biocompatibility, a critical factor that determines the safety and efficacy of these innovations. As devices increasingly interact with biological systems, the need for rigorous testing becomes paramount to prevent adverse reactions and ensure patient safety.
Biocompatibility testing encompasses a variety of methodologies, each aimed at assessing how materials perform in real-world clinical applications. With regulatory bodies like the FDA mandating comprehensive evaluations, understanding the nuances of biocompatibility is essential for manufacturers striving to navigate the complex landscape of medical device development.
This article delves into the significance of biocompatibility testing, the standards that govern it, the various testing methods employed, and the future challenges and trends shaping this vital field.
What is Biocompatibility Testing and Why is it Essential?
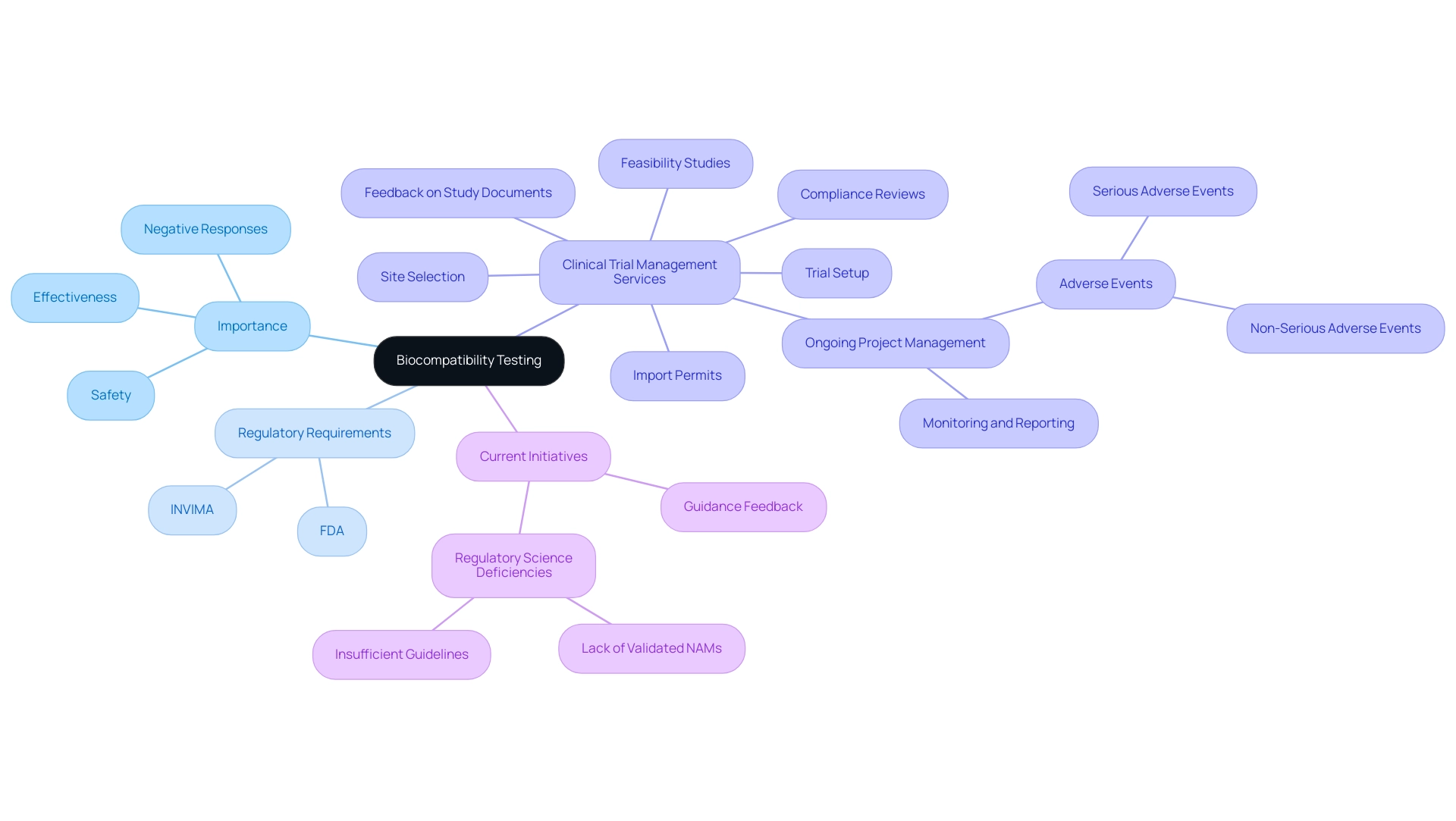
The biocompatibility testing of medical devices is an essential evaluation procedure that determines how suitable a healthcare instrument is with biological systems. This examination is crucial in evaluating the possibility of negative responses when a piece of equipment interacts with living tissue. Its importance cannot be exaggerated, as it plays a crucial role in ensuring the safety and effectiveness of healthcare instruments, thereby preventing potential complications that may arise from the materials utilized in their construction.
Regulatory bodies, especially the FDA and INVIMA, require thorough compatibility evaluations before the commercialization of medical instruments, establishing it as a crucial aspect of the medical instrument approval process. Our clinical trial management services encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Feedback on study documents to ensure adherence to country requirements
We also handle trial setup, import permits, and ongoing project management, which includes monitoring and reporting on study status, inventory, and both serious and non-serious adverse events.
Recently, the FDA approved two renal denervation devices designed to address high blood pressure, emphasizing the significance of thorough evaluation and comprehensive management in introducing new technologies to market. In light of recent developments, the FDA is currently soliciting feedback on draft guidance regarding biocompatibility assessments until November 18. This initiative reflects a commitment to refining assessment protocols and addressing the evolving landscape of regulatory requirements.
Sebastian Rodriguez-Elizalde, M.D., a member of the Scientific Advisory Board at Intellijoint Surgical, emphasizes that 'biocompatibility testing is fundamental to ensuring that healthcare instruments perform safely and effectively in clinical settings.' Additionally, the BTP has recognized significant regulatory science deficiencies, including:
- A lack of validated Names for compatibility evaluation
- Insufficient guidelines for assessing health technologies throughout their lifecycle
Through ongoing research, the program aims to address these gaps to improve safety evaluation methods while reducing burdens on the healthcare equipment sector.
Key Standards and Guidelines for Biocompatibility Testing
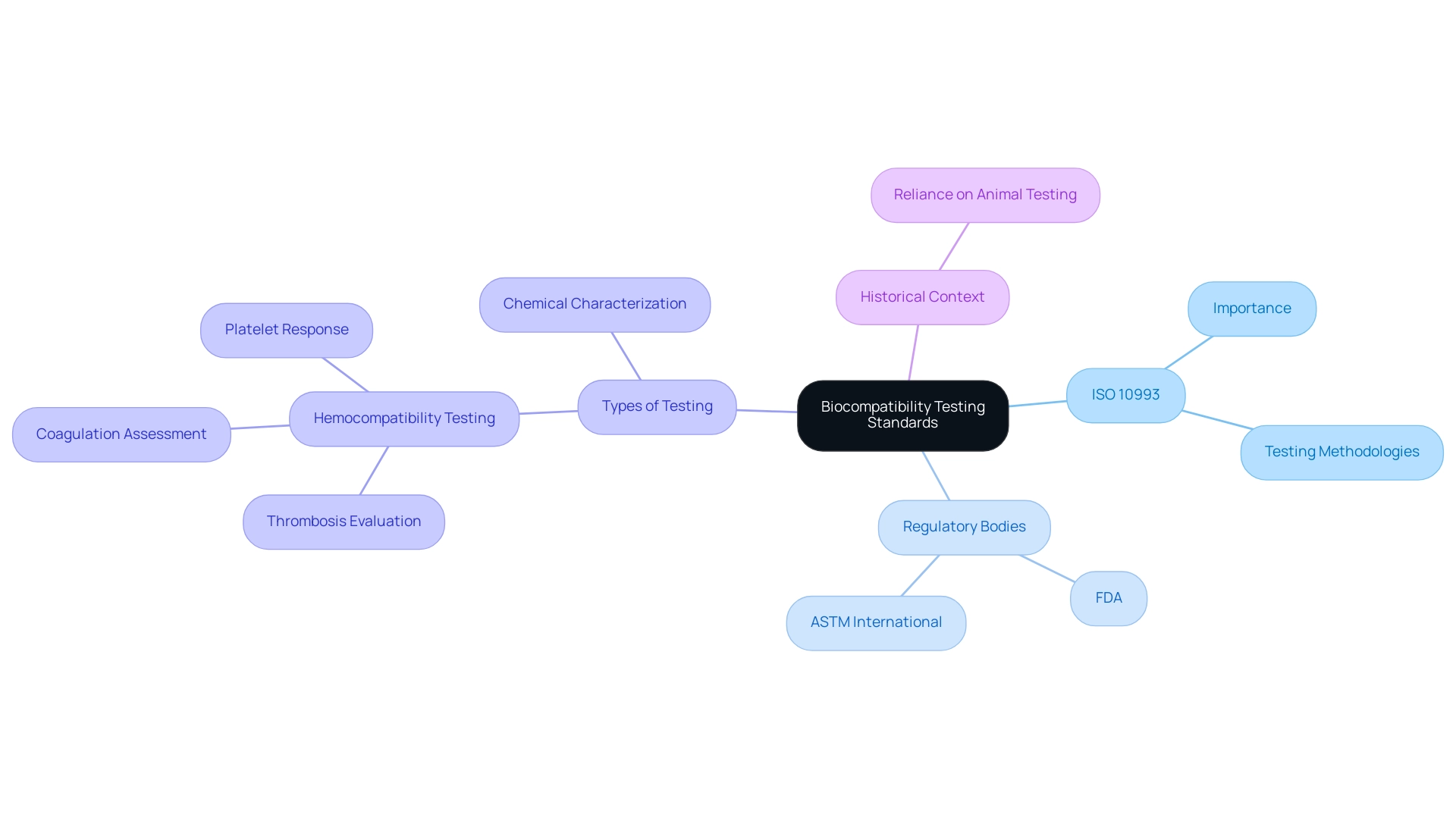
ISO 10993 serves as the fundamental standard for the biocompatibility testing of medical devices, outlining a thorough framework of evaluations aimed at measuring biological responses to materials. This standard underscores the necessity of examining both direct and indirect contact with bodily tissues and fluids, ensuring a holistic approach to safety. The evaluation of biocompatibility testing of medical devices acts as an essential link between laboratory assessments and real-world clinical applications, highlighting its importance in the medical product lifecycle.
In conjunction with ISO 10993, regulatory bodies such as the FDA and ASTM International provide critical guidelines that refine evaluation methodologies and protocols, adapting them to the latest scientific advancements. As of 2024, adherence to these standards not only ensures compliance with regulatory requirements but also bolsters the credibility of research outcomes. Such rigorous evaluation processes are pivotal for safeguarding patient health, particularly as manufacturers are tasked with including precautionary labeling regarding potential skin reactions for products intended for patients who may be unable to identify adverse effects.
A relevant example is hemocompatibility testing, which is crucial for healthcare products that contact blood, evaluating issues like thrombosis, coagulation, and platelet response, as outlined in ISO 10993-4. Furthermore, the chemical characterization of healthcare products is crucial for biocompatibility assessment, following various ISO standards, including ISO 10993-18. This process assists in comprehending the structural and functional characteristics of the apparatus and planning further evaluations.
As mentioned by Kerecman Mayers et al., despite significant advancements in the chemical industry, the healthcare equipment sector continues to depend extensively on conventional animal experimentation within ISO 10993 standards, emphasizing a field ready for future innovation.
Types of Biocompatibility Tests for Medical Devices
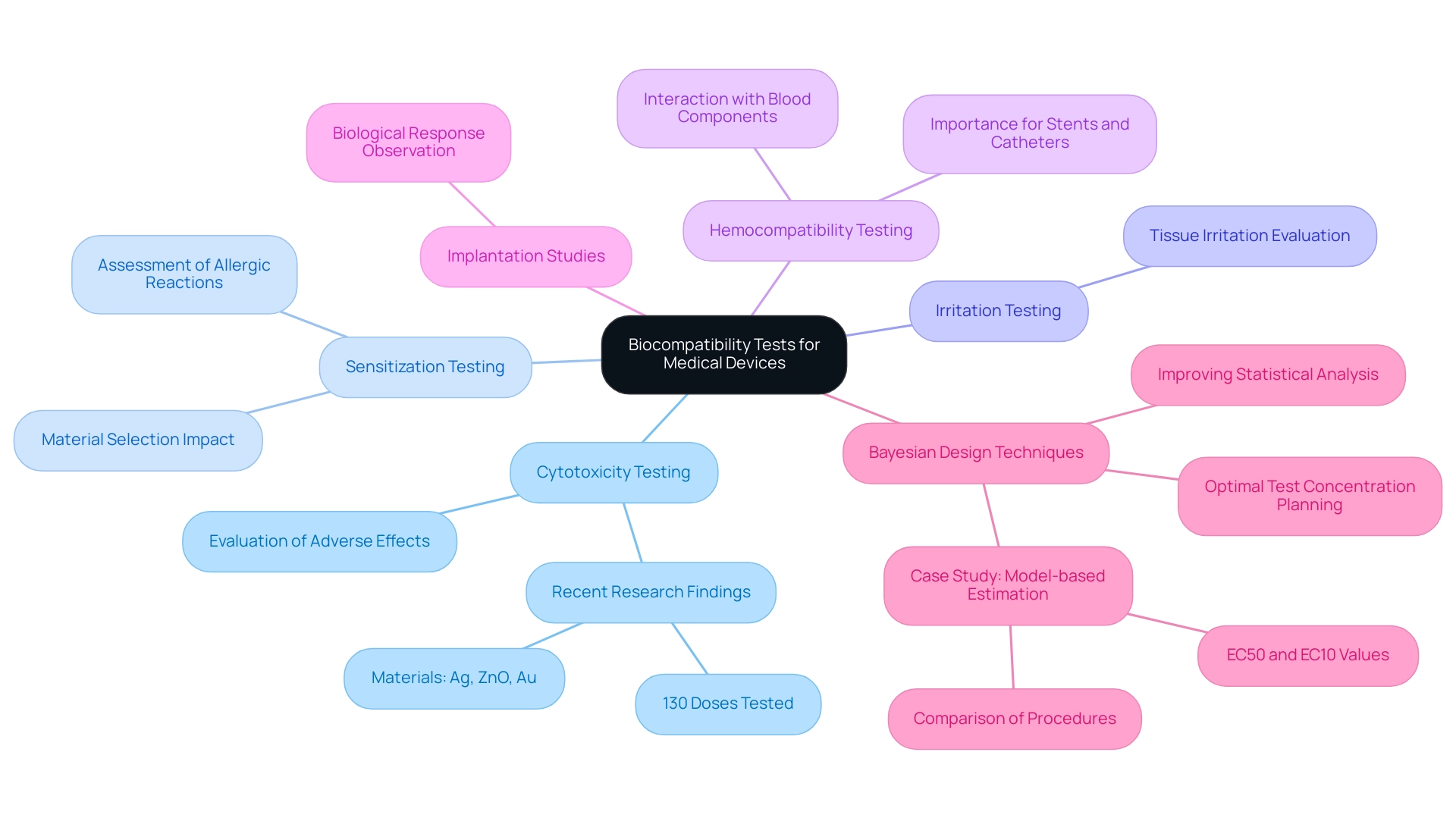
The biocompatibility testing of medical devices involves a wide range of approaches, each crucial for guaranteeing the safety and efficacy of materials in clinical uses. The following are key types of biocompatibility tests:
-
Cytotoxicity Testing: This test evaluates the potential adverse effects of materials on cultured cells, providing insights into their safety profile.
Recent research has shown that a total of 130 different doses of silver (Ag), zinc oxide (ZnO), and gold (Au) were tested across 20 studies, highlighting the extensive evaluation of these materials. This extensive testing highlights the significance of thorough cytotoxicity evaluations in determining the safety of healthcare product materials.
-
Sensitization Testing: This method assesses the likelihood of a material causing allergic reactions in living organisms, which is crucial for patient safety.
Significantly, the occurrence of allergic reactions linked to particular materials has led to heightened examination in the choice of materials for manufacturing.
-
Irritation Testing: This evaluation determines whether a product may cause irritation to tissue upon contact, thereby ensuring that materials used in medical equipment do not provoke adverse tissue responses.
-
Hemocompatibility Testing: This critical test analyzes the interactions between an instrument and blood components, especially important for products intended to come into contact with blood.
The findings from hemocompatibility tests are essential for items such as stents and catheters.
-
Implantation Studies: These involve placing instruments in animal models to observe the biological response over time, allowing for direct assessment of the instrument's performance in a living organism.
Each of these tests, particularly biocompatibility testing of medical devices, is integral to establishing an instrument's safety profile, ensuring compliance with necessary regulatory standards.
The importance of optimal test concentration planning is underscored by recent findings that suggest using Bayesian design techniques can enhance the quality of statistical analysis in toxicology, particularly when determining the lowest observed effect concentrations (EC50 and EC10) in cytotoxicity tests. As mentioned by Shawkey et al., 'similar results were observed for both Ag dosages,' reinforcing the importance of utilizing advanced methodologies in compatibility evaluation.
Furthermore, insights from the case study titled 'Model-based Estimation of Lowest Observed Effect Concentration' emphasize the advantages of Bayesian design techniques over traditional methods, highlighting the need for optimal test concentration planning to improve the quality of statistical analysis in toxicology.
Interpreting Biocompatibility Testing Results and Their Impact on Device Development
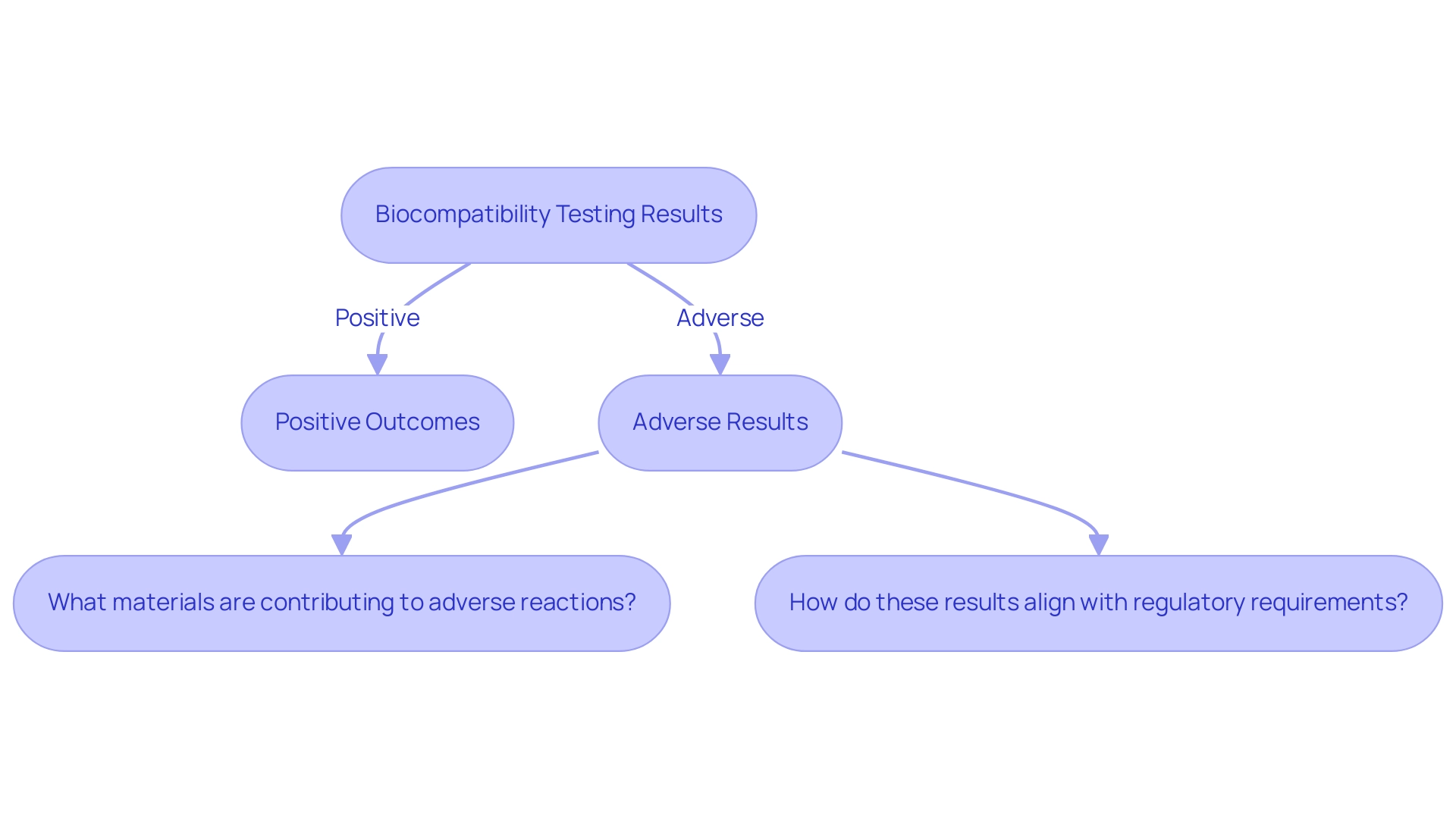
Analyzing the results of biocompatibility testing of medical devices is a vital part of confirming that medical instruments adhere to safety regulations. Positive outcomes typically signify a favorable biological response, while adverse results can trigger the need for further investigation or redesign. For instance, if cytotoxicity testing indicates significant cell death, it prompts researchers to explore alternative materials or revise designs to enhance safety.
Designers should ask specific questions during investigations, such as:
- 'What materials are contributing to adverse reactions?'
- 'How do these results align with regulatory requirements?'
Comprehending the intricacies of these results is crucial, as they directly impact the timeline and resources allocated to development.
Recent research on nanogenerator-based cardiovascular sensors emphasizes the need for biocompatibility testing of medical devices, as these devices necessitate high compatibility with living tissues to mitigate complications. The present focus on employing adaptable, biocompatible, and biodegradable substances highlights the significance of biocompatibility testing of medical devices for long-term evaluation. Nonetheless, the disparity in standardized accelerated methods for assessing compatibility remains a challenge in biocompatibility testing of medical devices.
With 15 years of experience in biocompatibility testing of medical devices at Nelson Labs, I can confirm that the results related to compatibility not only guide immediate design decisions but also influence the broader development strategy, potentially affecting timelines and regulatory pathways. As German chemists observed in 1789, comprehending the evolution of material use is crucial in this field, highlighting the need for strict evaluation standards.
Future Trends and Challenges in Biocompatibility Testing
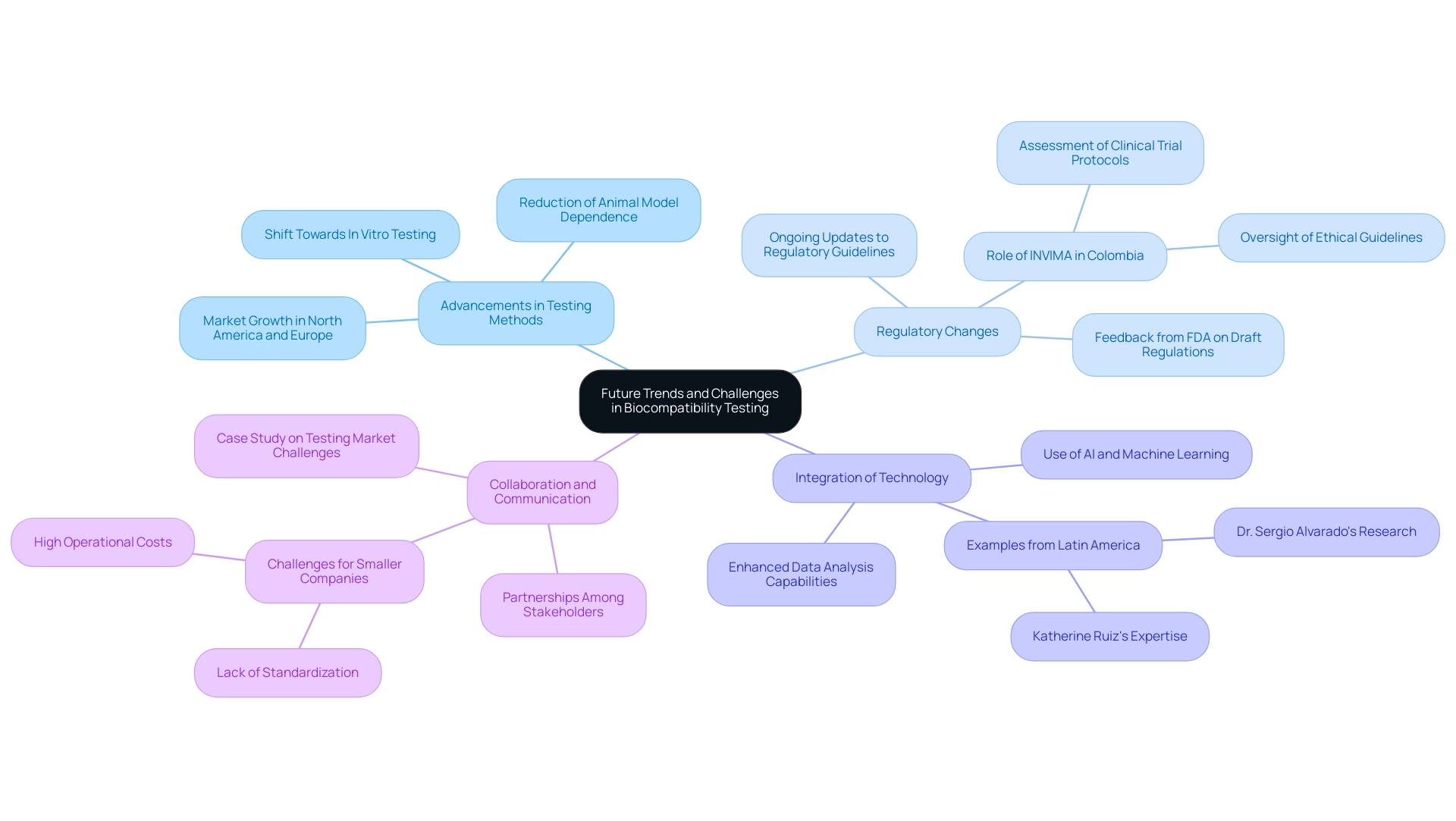
The landscape of biocompatibility testing is rapidly evolving, driven by several key trends and persistent challenges that shape the future of this critical field:
- Advancements in Testing Methods: The shift towards in vitro testing methodologies is significantly reducing dependence on animal models, thereby promoting more ethical research practices. This innovation not only aligns with global ethical standards but also facilitates quicker and more reliable biocompatibility testing of medical devices for material safety. According to recent market analysis, there is a projected significant growth in the biocompatibility testing of medical devices market, with a notable increase in demand across regions like North America and Europe.
- Regulatory Changes: As the healthcare equipment industry observes the introduction of novel materials and technologies, regulatory guidelines are continually being updated. This fluidity in regulations demands that researchers and manufacturers remain agile and well-informed, particularly in adapting their practices to meet the new compliance requirements for biocompatibility testing of medical devices. In Colombia, the INVIMA plays a crucial role in overseeing these changes, ensuring that medical devices comply with national standards. INVIMA's duties involve the assessment and endorsement of clinical trial protocols and overseeing adherence to ethical guidelines, which are crucial for the biocompatibility testing of medical devices. As Nick Paul Taylor noted, "The FDA is accepting feedback on the draft until Nov. 18," highlighting the ongoing dialogue between regulatory bodies and industry stakeholders.
- Integration of Technology: The incorporation of artificial intelligence and machine learning techniques into data analysis is poised to enhance both the accuracy and efficiency of biocompatibility testing of medical devices. By leveraging these advanced technologies, companies can streamline their evaluation processes and better interpret complex biological data, leading to more reliable outcomes. Dr. Sergio Alvarado’s focus on innovative medical research in Latin America exemplifies this trend, as does the expertise of Katherine Ruiz in regulatory affairs for medical devices and in vitro diagnostics in Colombia.
- Collaboration and Communication: Strengthening partnerships among various stakeholders, including manufacturers, regulatory bodies, and research institutions, is crucial for streamlining the evaluation process. Improved teamwork promotes better communication, which can result in greater adherence and a more unified strategy for biocompatibility testing of medical devices throughout the sector. However, challenges persist, especially for smaller companies encountering high operational costs and a lack of standardization in evaluation protocols, as emphasized in a recent case study on obstacles in the evaluation market.
These emerging trends signify a pivotal shift towards more efficient, ethical, and comprehensive biocompatibility testing of medical devices practices. Ongoing adaptation is essential as the industry navigates the rapid advancements in technology and regulatory landscapes. The comprehensive clinical trial management services, including feasibility studies, trial setup, and project management, are critical for ensuring that the biocompatibility testing of medical devices aligns with regulatory expectations and supports advances in medical device innovation.
Conclusion
Biocompatibility testing stands as a cornerstone in the development of medical devices, ensuring that these innovations are safe and effective for patient use. The rigorous methodologies employed in testing, ranging from cytotoxicity to hemocompatibility assessments, play a vital role in evaluating how materials interact with biological systems. Regulatory bodies like the FDA and INVIMA mandate these evaluations, reinforcing their importance in the device approval process. As highlighted in the article, advancements in testing methods and the integration of technology are paving the way for more ethical and efficient assessments, while ongoing regulatory updates necessitate that manufacturers remain agile and informed.
The future of biocompatibility testing is poised for transformation, driven by emerging trends such as the shift towards in vitro methodologies and the incorporation of artificial intelligence in data analysis. These developments not only enhance the accuracy of testing but also align with the growing emphasis on ethical standards in research. However, challenges persist, particularly for smaller companies navigating complex regulatory landscapes and high operational costs.
Ultimately, the commitment to rigorous biocompatibility testing is essential for safeguarding patient health and advancing medical device innovation. As the industry evolves, continuous collaboration among stakeholders will be critical in addressing these challenges and optimizing testing protocols. The importance of this field cannot be overstated, as it directly impacts the safety and efficacy of medical devices that are integral to modern healthcare.
Frequently Asked Questions
What is biocompatibility testing of medical devices?
Biocompatibility testing is an evaluation procedure that determines how suitable a healthcare instrument is with biological systems, assessing the potential negative responses when the device interacts with living tissue.
Why is biocompatibility testing important?
It is crucial for ensuring the safety and effectiveness of healthcare instruments, preventing potential complications arising from the materials used in their construction.
Which regulatory bodies require biocompatibility evaluations?
Regulatory bodies such as the FDA and INVIMA require thorough compatibility evaluations before the commercialization of medical instruments.
What services are included in clinical trial management related to biocompatibility testing?
Clinical trial management services include feasibility studies, site selection, compliance reviews, feedback on study documents, trial setup, import permits, and ongoing project management.
What recent developments have occurred regarding biocompatibility testing?
The FDA recently approved two renal denervation devices for high blood pressure and is soliciting feedback on draft guidance regarding biocompatibility assessments until November 18.
What standards guide biocompatibility testing?
ISO 10993 serves as the fundamental standard for biocompatibility testing, outlining evaluations to measure biological responses to materials.
What does ISO 10993 emphasize in biocompatibility testing?
It emphasizes examining both direct and indirect contact with bodily tissues and fluids, ensuring a holistic approach to safety.
What is hemocompatibility testing?
Hemocompatibility testing evaluates healthcare products that contact blood, focusing on issues like thrombosis, coagulation, and platelet response.
How does chemical characterization relate to biocompatibility assessment?
Chemical characterization is essential for understanding the structural and functional characteristics of healthcare products, guiding further evaluations.
What challenges does the healthcare equipment sector face regarding biocompatibility testing?
Despite advancements, the sector still relies heavily on conventional animal experimentation, indicating a need for innovation in testing methods.

