Overview
The article explores the factors contributing to the growth of clinical trials in Latin America, highlighting the region's diverse population, evolving regulatory frameworks, and cost advantages as key drivers. It supports this by detailing how local partnerships, demographic considerations, and technological advancements enhance research effectiveness, while also addressing challenges such as regulatory hurdles and the need for cultural competence in study designs.
Introduction
As the landscape of global clinical research continues to evolve, Latin America is emerging as a pivotal hub for clinical trials, driven by its unique demographic and epidemiological characteristics. The region's diverse population not only provides a rich participant base but also presents an array of health challenges that necessitate tailored research efforts. Countries such as Brazil, Mexico, and Argentina are at the forefront, showcasing favorable regulatory frameworks and cost advantages that make them attractive destinations for pharmaceutical companies.
However, navigating the complexities of conducting clinical trials in this region involves addressing various challenges, including:
- Regulatory hurdles
- The need for local partnerships
This article delves into the factors propelling Latin America’s growth in clinical research, examines the demographic and epidemiological considerations that shape trial designs, and outlines best practices for enhancing trial success, ultimately positioning the region as a significant player in the global healthcare landscape.
The Emergence of Latin America as a Hub for Clinical Trials
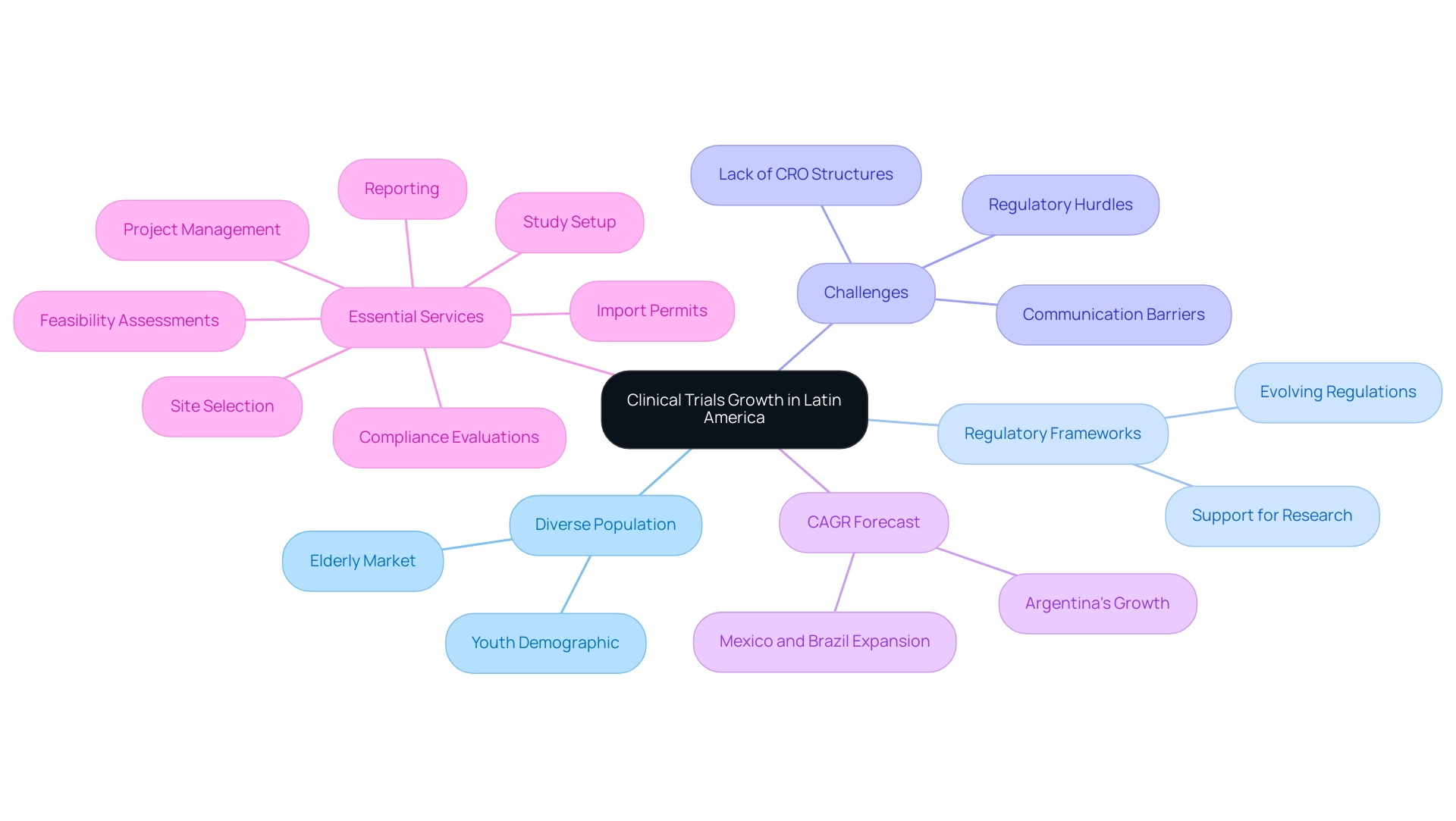
The area is quickly becoming a crucial center for medical studies, which is contributing to the clinical trial growth in Latin America, mainly because of its diverse population that provides a rich and varied participant base for research. Countries such as Brazil, Mexico, and Argentina not only present evolving regulatory frameworks that support research but also exhibit a high prevalence of diseases pertinent to global health challenges. As of 2023, the area represented 2.1% of the global research trials market, with Mexico and Brazil ready for significant expansion, which is indicative of the clinical trial growth in Latin America due to their cost benefits in comparison to advanced economies.
Furthermore, roughly 30% of the population in the region is under 14 years old, indicating a growing elderly patient demographic and a potentially large new drug market. The area is experiencing a substantial rise in skilled experts and committed research organizations, promoting an atmosphere favorable for progressing medical research. However, U.S. Medtech companies face challenges such as regulatory hurdles, communication barriers, and the lack of CRO corporate structures in Latin America, which complicates collaboration.
In this context, partnerships with local entities like bioaccess® become essential to bridge these gaps. Experts have forecasted that Argentina will record the highest compound annual growth rate (CAGR) from 2024 to 2030, indicating a significant clinical trial growth in Latin America, and highlighting its potential as a strategic site for multinational pharmaceutical companies looking to carry out research both efficiently and effectively. Furthermore, extensive research study management services, including:
- feasibility assessments
- site selection
- compliance evaluations
- study setup
- import permits
- project management
- reporting
are essential for navigating the changing landscape.
As these dynamics continue to evolve, clinical trial growth in Latin America is set to make the region a more appealing destination for research initiatives in healthcare, driving global health enhancement through international collaboration and innovation in Medtech.
Navigating Challenges in Clinical Trial Implementation in Latin America
The complex range of difficulties in carrying out medical studies is a significant challenge to clinical trial growth in Latin America, primarily due to the intricate regulatory frameworks that vary significantly between nations. For example, while Chile boasts retention rates exceeding 85% in clinical studies, surpassing global averages, the average time for regulatory approval can vary significantly, often leading to delays that disrupt study timelines. Navigating these regulatory hurdles is informed by the expertise of local authorities like INVIMA, Colombia's National Food and Drug Surveillance Institute, which plays a crucial role in ensuring compliance and overseeing the marketing of health products.
This is particularly important given its classification as a Level 4 health authority by PAHO/WHO, which underscores its competence in regulatory functions essential for safeguarding the quality and efficacy of medical devices. However, operational difficulties persist, including inadequate infrastructure, limited access to advanced technology, and inconsistencies in patient recruitment efforts, all of which can hinder clinical trial growth in Latin America. Resource constraints—such as insufficient funding and a shortage of trained personnel—pose considerable barriers to the effective execution of clinical trial growth in Latin America.
The case study on cannabis seizures in the region emphasizes further regulatory obstacles; persistent problems with unlawful cultivation and trafficking can complicate the regulatory environment, affecting the viability of performing research. Expert opinions suggest that overcoming these challenges requires a strategic understanding of the local landscape. As Julio G. Martinez-Clark, CEO of bioaccess®, aptly stated, 'By combining regulatory speed, ethical oversight, and a robust healthcare system, Chile is not just participating in the medical device research landscape; it's actively shaping its future in Latin America.'
This proactive stance exemplifies the potential for collaboration and innovation within the region, highlighting the importance of clinical trial growth in Latin America despite the overarching challenges. Tackling these problems is essential for researchers seeking to maneuver through the intricacies of medical studies effectively. Moreover, our extensive research management services include:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting
Ensuring that all elements of the research process are meticulously managed.
Experts such as Ana Criado, Director of Regulatory Affairs, and Katherine Ruiz, a specialist in Regulatory Affairs for Medical Devices and In Vitro Diagnostics, provide invaluable knowledge to simplify these processes and improve the success of research studies in the regions.
Demographic and Epidemiological Considerations for Clinical Trials
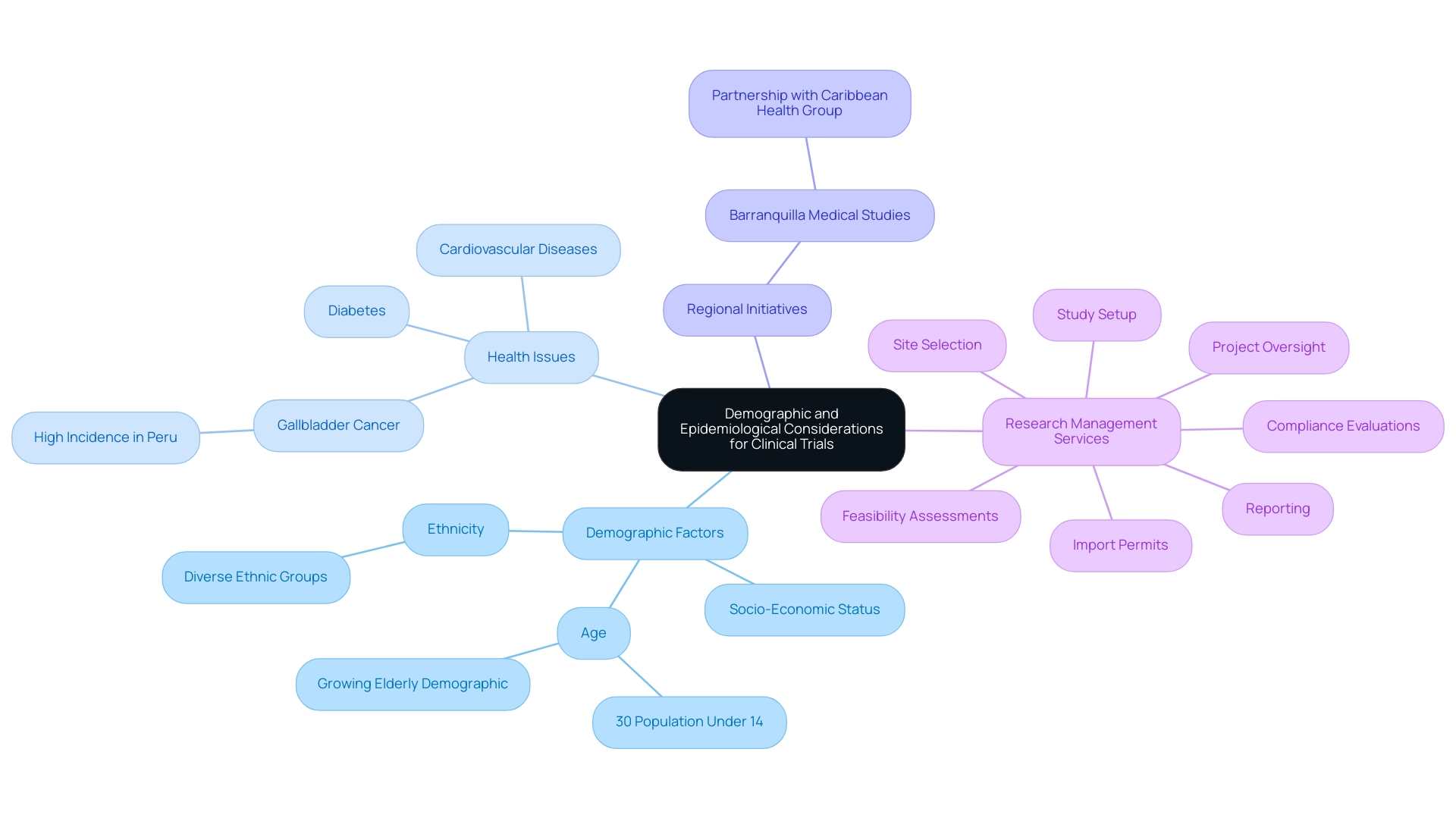
Demographic elements, such as age, ethnicity, and socio-economic status, play a crucial role in influencing study design and participant recruitment throughout Latin America. This region boasts a diverse population comprising numerous ethnic groups, each exhibiting distinct health profiles and varying disease prevalence. For instance, the notably high occurrence of gallbladder cancer in Peru exemplifies specific regional health challenges that necessitate tailored trials to address these conditions effectively.
Furthermore, current epidemiological data underscores the pressing need for clinical research focused on prevalent conditions such as diabetes and cardiovascular diseases, which significantly impact public health. As noted by Fabio Franke from Oncosite, 'the potential for patient recruitment and cost advantages in the Latin region significantly contribute to the clinical trial growth in Latin America, making it an attractive area for industry consideration.' Additionally, with approximately 30% of Latin America's population under 14 years old, there is a critical need to anticipate the future growth of the elderly demographic, which will influence the design of relevant medical interventions.
The partnership between bioaccess® and Caribbean Health Group, supported by Colombia's Minister of Health, aims to establish Barranquilla as a premier location for medical studies, thereby contributing to the clinical trial growth in Latin America and enhancing the region's attractiveness. This initiative, in conjunction with Global Care Clinical Trials collaborating with bioaccess® to enhance recruitment durations and retention percentages, underscores the extensive research management services offered, which are essential for supporting clinical trial growth in Latin America, including:
- feasibility assessments
- site selection
- compliance evaluations
- study setup
- import permits
- project oversight
- reporting
Furthermore, the wider context of health and regulatory issues, demonstrated by the recorded cannabis confiscations in the area, highlights the difficulties encountered in carrying out research studies.
The function of INVIMA, Colombia's National Food and Drug Surveillance Institute, is essential in monitoring regulatory activities and guaranteeing adherence to health standards, which affects the operational environment for research studies. By comprehending these demographic dynamics and the surrounding environment, researchers can customize their studies more effectively, enhancing participant engagement and ensuring a more precise representation in research. This approach not only addresses the immediate health concerns of the population but also aligns with the anticipated growth in the elderly patient demographic, ultimately contributing to job creation, economic growth, and improved healthcare outcomes in the region.
Best Practices for Enhancing Clinical Trial Success in Latin America
To enhance the success of clinical studies in Latin America, particularly in Colombia, researchers should implement several key best practices:
- Building Local Partnerships: Collaborating with local institutions and researchers is essential for improving participant recruitment and fostering community trust. Colombia has recognized these benefits and has an ambitious science, technology, and innovation plan for 2022–2031 to become a knowledge economy. These partnerships can facilitate a deeper understanding of local health dynamics, thus enhancing the effectiveness of the study.
- Cultural Competence: Recognizing the cultural nuances and health beliefs prevalent within the population is crucial for effective communication and participant engagement. This understanding can help mitigate barriers to participation and ensure that study protocols are culturally sensitive. For instance, the challenges related to illegal cannabis cultivation and trafficking in the region highlight the need for cultural awareness in addressing community concerns.
- Streamlined Regulatory Processes: Engaging with regulatory bodies early in the design phase can significantly ease the navigation of regulatory approvals. In Colombia, the total IRB/EC and MoH (INVIMA) review takes only 90-120 days, which is considerably faster than many regions. This proactive approach helps ensure compliance with local standards and accelerates the commencement of experiments. Significantly, 52 percent of global studies take place outside the U.S., which highlights the necessity of effective regulatory navigation to support clinical trial growth in Latin America.
- Training and Capacity Building: Investing in the training of local staff is vital to equip them with the necessary skills to meet the demands of contemporary research. A well-trained workforce can enhance data quality and research integrity.
- Utilizing Technology: Leveraging digital tools for data collection and patient engagement can enhance the efficiency and reach of medical studies. Technologies such as electronic data capture (EDC) systems and mobile health applications improve participant monitoring and data accuracy. Furthermore, the experience of Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, during the first human test of bioaccess® in Colombia illustrates the practical benefits of these strategies. By efficiently applying these methods, researchers can significantly enhance the results of their studies in the region, aligning with the larger goals of promoting global health research. Significantly, carrying out medical studies in Colombia can result in cost reductions of over 30% relative to North and South regions or Western Europe, and the R&D tax benefits available, including a 100% tax write-off and considerable government funding, further improve the appeal of Colombia as a research destination.
Future Perspectives: The Growth Trajectory of Clinical Trials in Latin America
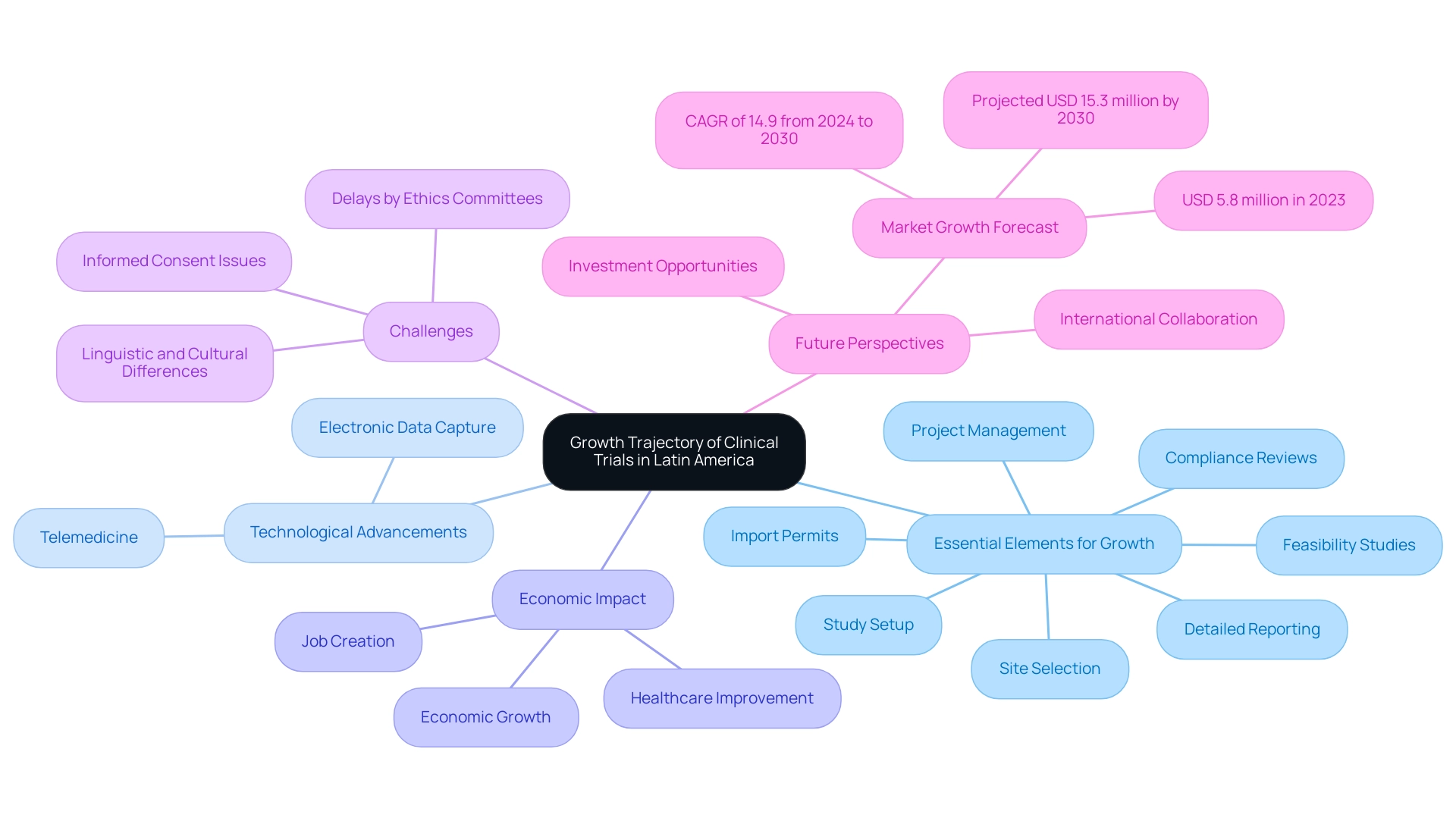
The perspective for medical studies indicates that there is significant clinical trial growth in Latin America, propelled by various essential elements ready to encourage expansion. Our extensive research management services include:
- Feasibility studies
- Site selection
- Compliance reviews that ensure adherence to country requirements
- Study setup
- Import permits
- Project management
- Detailed reporting—crucial elements that enhance operational efficiency.
Technological advancements, particularly in telemedicine and electronic data capture, are set to revolutionize research methodologies, enhancing efficiency and patient engagement.
In 2023, the research matching software market in Latin America produced USD 5.8 million, with forecasts indicating an increase to USD 15.3 million by 2030, demonstrating a compound annual growth rate (CAGR) of 14.9% from 2024 to 2030. This growth is indicative of the increasing number of research organizations entering the market, fostering both competition and innovation. Furthermore, regional regulatory bodies are undertaking significant efforts to harmonize standards across countries, facilitating smoother implementations.
The impact of medtech research studies extends beyond investigation, contributing to local economies through job creation, economic growth, and healthcare improvement, while also driving international collaboration. However, challenges remain, particularly with delays by ethics committees often stemming from issues with informed consent, which can be mitigated by addressing linguistic and cultural differences. As the need for varied research participant groups grows, clinical trial growth in Latin America is making the region a more appealing location for pharmaceutical firms and scholars.
This trend is likely to drive further investment and opportunities for medical advancements, with clinical trial growth in Latin America positioning the region as a critical participant in the global research landscape. Anil Kumar P., Research Manager in Healthcare, emphasizes that high-quality research is essential for supporting informed decision-making, highlighting that technological advancements will play a vital role in the growth of clinical trials in Latin America.
Conclusion
Latin America is emerging as a pivotal hub for clinical trials, driven by its diverse population and significant health challenges that necessitate tailored research efforts. The region's favorable regulatory frameworks, alongside cost advantages, position countries like Brazil, Mexico, and Argentina as attractive destinations for pharmaceutical companies. However, navigating the complexities of conducting clinical trials in this region requires addressing various challenges, including regulatory hurdles and the need for local partnerships.
The article highlights the demographic and epidemiological factors that shape clinical trial designs, emphasizing the importance of understanding local health dynamics and cultural nuances. By implementing best practices such as:
- Building local partnerships
- Fostering cultural competence
- Utilizing technology
researchers can enhance participant engagement and trial success. Furthermore, the optimistic future trajectory for clinical trials in Latin America is underscored by technological advancements and a growing number of clinical research organizations entering the market.
In conclusion, the potential for Latin America to become a significant player in the global clinical research landscape is evident. By leveraging its rich participant base and addressing the unique challenges present, the region can drive innovations in healthcare and improve global health outcomes. As the demand for diverse clinical trial populations increases, stakeholders must continue to invest in local infrastructure and expertise, ensuring that Latin America remains at the forefront of clinical research initiatives.
Frequently Asked Questions
Why is Latin America becoming a crucial center for medical studies?
Latin America is becoming a crucial center for medical studies due to its diverse population, which provides a rich participant base for research, along with evolving regulatory frameworks and a high prevalence of diseases relevant to global health challenges.
What percentage of the global research trials market does Latin America represent as of 2023?
As of 2023, Latin America represents 2.1% of the global research trials market.
Which countries in Latin America are poised for significant clinical trial growth?
Mexico and Brazil are identified as countries ready for significant clinical trial growth in Latin America.
What demographic trend in Latin America contributes to the potential for a new drug market?
Approximately 30% of the population in the region is under 14 years old, indicating a growing elderly patient demographic and a potentially large new drug market.
What challenges do U.S. Medtech companies face in Latin America?
U.S. Medtech companies face regulatory hurdles, communication barriers, and the lack of corporate research organization (CRO) structures in Latin America, complicating collaboration.
What services are essential for navigating the clinical trial landscape in Latin America?
Essential services include feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, and reporting.
Which country in Latin America is forecasted to have the highest compound annual growth rate (CAGR) from 2024 to 2030?
Argentina is forecasted to record the highest compound annual growth rate (CAGR) from 2024 to 2030.
What operational difficulties hinder clinical trial growth in Latin America?
Operational difficulties include inadequate infrastructure, limited access to advanced technology, inconsistencies in patient recruitment efforts, insufficient funding, and a shortage of trained personnel.
How does the regulatory environment impact clinical trials in Latin America?
The regulatory environment presents challenges due to varying frameworks between nations, with potential delays in approval processes that disrupt study timelines.
What is the role of local authorities like INVIMA in the clinical trial process?
Local authorities like INVIMA play a crucial role in ensuring compliance and overseeing the marketing of health products, which is essential for safeguarding the quality and efficacy of medical devices.
What expert insights are available to navigate the complexities of clinical trials in Latin America?
Experts such as Ana Criado and Katherine Ruiz provide invaluable knowledge to simplify the processes and improve the success of research studies in the region.

