Overview
The article explores the emergence of Latin America as a significant destination for clinical trials, highlighting its diverse patient population, lower operational costs, and supportive regulatory environment as key advantages. It also addresses the challenges faced by Medtech companies, such as regulatory hurdles and language barriers, while showcasing successful partnerships and advancements that position the region competitively in the global clinical research landscape.
Introduction
Latin America is rapidly establishing itself as a key player in the global clinical trial landscape, driven by a confluence of factors that make it an attractive destination for medical research. With its diverse patient populations, lower operational costs, and evolving regulatory frameworks, countries such as Brazil, Mexico, and Colombia are emerging as hotspots for innovative clinical studies.
However, navigating this promising terrain comes with its own set of challenges, including regulatory complexities and communication barriers that can hinder collaboration with international sponsors.
This article delves into the dynamics shaping Latin America's rise in the clinical trial sector, highlighting the opportunities and obstacles that define this vibrant market, while emphasizing the importance of strategic partnerships and technological advancements in enhancing trial efficiency and accessibility.
The Rise of Latin America as a Clinical Trial Destination
Latin America has emerged as one of the clinical trial hotspots in Latin America, driven by several key factors. The region boasts a diverse patient population, essential for obtaining varied and representative data. Additionally, the operational expenses related to carrying out tests in countries like Brazil, Mexico, and Argentina are notably lower than in other regions.
These benefits are enhanced by a developing regulatory framework that actively supports medical studies. However, Medtech companies encounter significant challenges, including:
- Regulatory hurdles
- Professionalism issues
- Language barriers
- Fragmented resources
These challenges can impede seamless communication and collaboration with American clinical research clients. Each nation has developed robust healthcare infrastructures and a growing number of specialized research centers, establishing them as clinical trial hotspots in Latin America that are attractive locations for global sponsors.
Notably, Argentina's global innovation index score has improved from 2011 to 2023, reflecting the country's commitment to fostering innovation in healthcare. Successful partnerships, like the one between Greenlight Guru and bioaccess™, demonstrate the potential to expedite Medtech innovations and studies in Latin America. Furthermore, initiatives backed by local health agencies, such as the partnership between bioaccess™ and Caribbean Health Group, establish Barranquilla as a prominent location for medical studies.
The impressive results from GlobalCare Clinical Trials, in partnership with bioaccess™, demonstrate a remarkable over 50% reduction in recruitment time and a 95% retention rate. As a testament to this trend, Flow-FX's first-in-human study for its innovative Flow-Screw medical device, delivering intraosseous antibiotics in Colombia, led by Dr. Carlos Severini, exemplifies the success of these strategies. With these components merged and acknowledging current obstacles, the region is positioned as a competitive environment for carrying out studies, particularly as clinical trial hotspots in Latin America that draw considerable investment and focus from the global scientific community.
Identifying Key Hotspots for Clinical Trials in Latin America
Brazil, Mexico, and Colombia are recognized as clinical trial hotspots in Latin America, each offering distinct benefits that attract research efforts. Brazil stands out due to its robust healthcare infrastructure and diverse patient demographics, which facilitate the recruitment of varied patient populations. According to recent reports, Brazil continues to lead the region in research activity, with significant growth observed in Phase III studies, which are essential for assessing the effectiveness of new treatments.
In fact, the clinical trial hotspots in Latin America are anticipated to experience significant growth in the research market, especially in Phase III, fueled by the expansion of Contract Research Organizations (CROs) and positive patient-physician relationships. Mexico, in contrast, features a favorable regulatory environment that promotes pharmaceutical research, leading to a significant number of studies involving human subjects. In 2023, Mexico was emphasized for its strategic positioning in the research market, especially because of its robust partnership between patients and doctors.
As Dr. Larissa Aviles-Santa, Director of the Division of Clinical and Health Services Research, observes, 'The success of medical studies in these regions depends on the collaborative efforts between researchers and local healthcare providers.' Meanwhile, Colombia has emerged as a clinical trial hotspot in Latin America, owing to its supportive governmental policies and competitive costs. The nation has shown a dedication to promoting medical studies, which is evident in the growing number of experiments being carried out.
Significantly, Dr. John B. Simpson, CEO of Avinger, highlighted the satisfactory experience conducting OCT-guided atherectomy trials at a site in Cali, Colombia, demonstrating the effective partnership with LATAM CRO Experts. Thorough research management services, including feasibility studies, site selection, compliance reviews, setup, and project management, are essential for navigating the complexities of medical research in these regions. Additionally, INVIMA, Colombia's National Food and Drug Surveillance Institute, plays a vital role in overseeing research studies, ensuring adherence to national standards and facilitating the importation of investigational devices.
These areas not only offer access to large patient groups but also gain from a pool of skilled experts proficient at managing studies efficiently. As the landscape of medical research evolves, staying informed about clinical trial hotspots in Latin America and leveraging market intelligence is crucial for organizations aiming to maximize their recruitment strategies.
Navigating Regulatory Challenges in Latin American Clinical Trials
Navigating the regulatory environment in clinical trial hotspots in Latin America requires a comprehensive understanding of both local laws and international standards, as each nation has its appointed regulatory body supervising research studies. In Brazil, this responsibility falls to ANVISA, while COFEPRIS serves a similar function in Mexico. Researchers must prioritize adherence to Good Clinical Practice (GCP) guidelines and local regulations to reduce the risk of delays or complications in the research process.
Working with local specialists and regulatory advisors is crucial, as their insights can aid in smoother navigation through these regulatory challenges, ensuring studies not only follow ethical standards but also prioritize participant safety. In Colombia, INVIMA functions as the national regulatory body, categorized as a Level 4 health authority by PAHO/WHO, supervising medical device regulation and clinical study compliance. Our service capabilities encompass feasibility studies and site selection, which are essential for successful project initiation.
Additionally, we offer extensive project management and monitoring services, ensuring that all facets of the study are conducted efficiently and in compliance with local regulations. With the Colombian government striving to improve its medical investigation environment to meet international standards by 2031, the potential for growth in the region is substantial. Experts predict that Colombia could emerge as one of the clinical trial hotspots in Latin America, witnessing over 100 new clinical trials annually and generating close to $500 million in economic gains per year.
However, barriers persist, such as the weak financial management of study groups and an inadequate regulatory context. Henry L. Gómez from Oncosalud-AUNA notes, "the weak financial management of study groups and inadequate regulatory context are barriers found in Latin America; however, medical oncologists perceive the real potential of the region." Furthermore, the importance of carrying out local studies in low and middle-income countries has been emphasized, particularly its effect on altering healthcare practices, underscoring the necessity for access to academic study groups.
Our reporting processes ensure that study status, inventory, and adverse events are meticulously documented, providing transparency and accountability throughout the study.
Leveraging Technology for Enhanced Clinical Trial Efficiency
The incorporation of technology into medical studies has led to significant changes in the research environment. Tools such as electronic data capture (EDC) systems, remote monitoring applications, and patient engagement platforms facilitate real-time data collection and enhance communication with participants. Recent advancements in EDC systems are streamlining workflows and improving data accuracy, which is critical given that only 5% of patients completed all seven modules of post-discharge requirements despite 84% completing at least one additional module.
Moreover, continuous initiatives to implement Fast Healthcare Interoperability Resources (FHIR) standards are essential for improving electronic healthcare information exchange, thus aiding the incorporation of technology in medical studies. Leveraging big data analytics can significantly enhance patient recruitment and retention strategies by enabling researchers to identify and engage eligible candidates more effectively. The emergence of telemedicine in medical studies is especially significant, as it enables researchers to connect with broader patient groups, including individuals in distant locations, thereby enhancing participation rates.
As emphasized by Orri and colleagues in their 2014 REMOTE study, the use of e-technologies—ranging from social media to online health forums—has proven effective in recruiting participants and managing their involvement through Internet-based consent and surveys. Additionally, the case study titled 'Future Directions for E-Technologies in Clinical Trials' underscores the necessity for meticulous planning and collaboration among multidisciplinary teams to address the challenges associated with integrating e-technologies. These developments emphasize the necessity for medical researchers to adopt innovative digital solutions to meet the growing demand for new evidence.
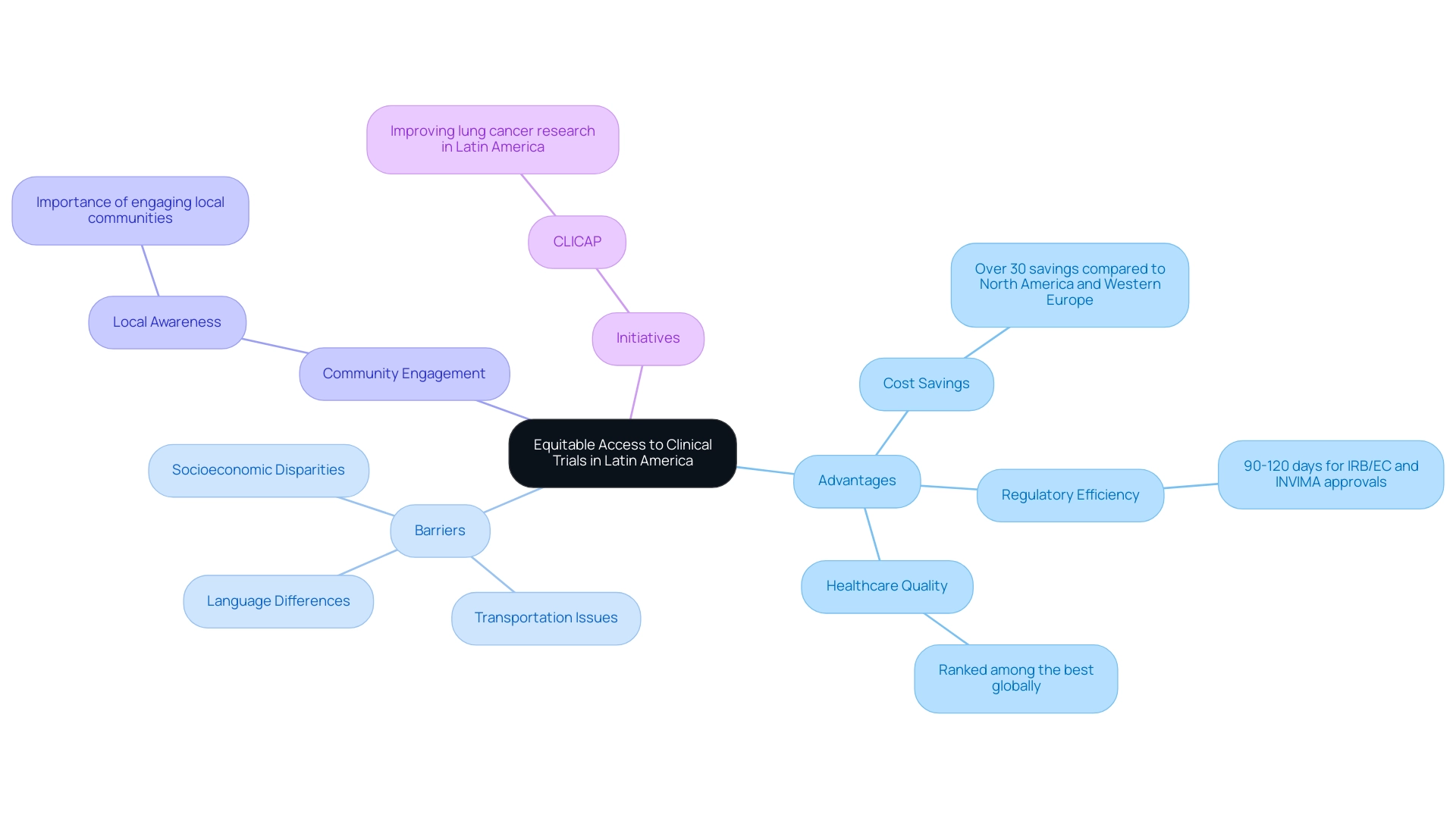
Ensuring Equitable Access to Clinical Trials in Latin America
Fair access to research studies is crucial for promoting medical advancements and improving health results among various communities. In Latin America, where regulatory timelines and political instability present significant challenges, it is crucial to implement effective strategies in clinical trial hotspots in Latin America that promote inclusivity.
Colombia is recognized as one of the clinical trial hotspots in Latin America, standing out as a leading destination for first-in-human studies due to its competitive advantages, including:
- Cost savings of over 30% compared to North America and Western Europe
- Rapid regulatory review processes of 90-120 days for IRB/EC and INVIMA approvals
- A high-quality healthcare system ranked among the best globally
Furthermore, investments in R&D benefit from substantial tax incentives, including a 100% tax deduction and various credits, making it economically advantageous for sponsors. Key barriers to participation, such as transportation issues, language differences, and socioeconomic disparities, must be addressed proactively. Engaging local communities and stakeholders is essential to raise awareness about the significance of clinical trials, particularly among underrepresented groups.
Initiatives such as the American Consortium for the Investigation of Lung Cancer (CLICAP), established in 2011, exemplify the potential of community involvement in improving recruitment efforts. As CLICAP states, "Our work aims to enhance lung cancer studies in South America," emphasizing the consortium's dedication to this cause. Furthermore, the involvement of experts like Juliana Giacomazzi from the Latin American Cooperative Oncology Group (LACOG) highlights collaborative efforts to tackle these challenges.
The rigorous ICH/GCP certification process that hospitals must undergo before conducting medical studies ensures high-quality standards. The development of culturally sensitive educational materials and the provision of support services can significantly boost recruitment efforts. By ensuring that medical studies reflect the diverse demographics they intend to serve, we can cultivate a more inclusive environment that benefits all participants.
Moreover, media attention from Clinical Leader highlights the increasing acknowledgment of research studies in South America, underscoring the significance of clinical trial hotspots in Latin America as part of a strong research ecosystem. Notably, Brazil's contribution of 1,561 citable documents in 2020, representing 45.8% of the region's total, underscores the need for ongoing efforts to ensure equitable access to clinical trials across Latin America.
Conclusion
Latin America is emerging as a significant player in the global clinical trial landscape, driven by its diverse patient populations, lower operational costs, and supportive regulatory frameworks. Countries such as Brazil, Mexico, and Colombia are at the forefront, each offering unique advantages that enhance their attractiveness for clinical research. However, the journey is not without its challenges, including regulatory complexities and communication barriers that require careful navigation.
The importance of strategic partnerships and technological advancements cannot be overstated. Collaborative efforts among local healthcare providers and international sponsors are essential for overcoming obstacles and maximizing the potential of the region. Moreover, integrating technology into clinical trials has proven to enhance efficiency, improve patient engagement, and streamline data collection, ultimately leading to more successful outcomes.
As Latin America continues to develop its clinical research capabilities, the focus on equitable access remains paramount. Addressing barriers to participation and fostering community engagement will ensure that clinical trials reflect the diverse demographics they aim to serve. By prioritizing inclusivity and leveraging the region's strengths, the future of clinical trials in Latin America appears not only promising but also pivotal for advancing global medical research. The potential for growth and innovation in this dynamic landscape is substantial, positioning Latin America as a competitive and appealing destination for clinical trials on the world stage.
Frequently Asked Questions
Why is Latin America considered a hotspot for clinical trials?
Latin America is recognized as a hotspot for clinical trials due to its diverse patient population, lower operational expenses in countries like Brazil, Mexico, and Argentina, and a developing regulatory framework that supports medical studies.
What challenges do Medtech companies face in Latin America?
Medtech companies encounter challenges such as regulatory hurdles, professionalism issues, language barriers, and fragmented resources, which can hinder effective communication and collaboration with American clinical research clients.
What factors contribute to Brazil's status as a clinical trial hotspot?
Brazil's robust healthcare infrastructure and diverse patient demographics facilitate the recruitment of varied patient populations, leading to significant research activity, particularly in Phase III studies.
How does Mexico support clinical trials?
Mexico has a favorable regulatory environment that promotes pharmaceutical research, resulting in a high number of studies involving human subjects and strong partnerships between patients and healthcare providers.
What role does Colombia play in the clinical trial landscape?
Colombia has emerged as a clinical trial hotspot due to supportive governmental policies, competitive costs, and a growing number of experiments being conducted, backed by effective partnerships with local CROs.
What is the significance of partnerships in conducting clinical trials in Latin America?
Successful partnerships between researchers and local healthcare providers are crucial for the success of medical studies in Latin America, as they enhance collaboration and facilitate recruitment.
What are some successful examples of clinical trials in Latin America?
Successful examples include the partnership between Greenlight Guru and bioaccess™, which expedited Medtech innovations, and Flow-FX's first-in-human study in Colombia, demonstrating effective strategies in clinical trials.
How does INVIMA contribute to clinical trials in Colombia?
INVIMA, Colombia's National Food and Drug Surveillance Institute, oversees research studies, ensuring adherence to national standards and facilitating the importation of investigational devices.
What is expected for the future of clinical trials in Latin America?
The clinical trial hotspots in Latin America are anticipated to experience significant growth, especially in Phase III studies, driven by the expansion of Contract Research Organizations (CROs) and improved patient-physician relationships.

