Overview
Clinical trial innovation for devices in Argentina plays a crucial role in enhancing efficiency, safety, and effectiveness through adaptive study designs and the integration of digital health technologies. This article underscores the significance of these innovations by illustrating how they:
- Streamline research processes
- Improve patient engagement
- Facilitate real-time data collection
Such advancements ultimately lead to better healthcare outcomes and expedite market access for new medical devices, highlighting the transformative potential of these strategies in the Medtech landscape.
Introduction
In the ever-evolving landscape of medical technology, clinical trial innovation stands at the forefront, shaping the future of healthcare. As the demand for efficient and effective medical devices surges, the methodologies employed in clinical trials are undergoing a transformative shift. This article delves into the key innovations redefining clinical trials for medical devices, from adaptive trial designs that allow for real-time adjustments to the integration of digital health technologies that enhance patient engagement. By exploring the implications of these advancements, particularly in the context of Argentina's burgeoning Medtech sector, a clearer picture emerges of how innovative trial methodologies are not only accelerating development timelines but also improving patient outcomes. With the landscape becoming increasingly competitive, understanding these dynamics is crucial for stakeholders aiming to navigate the complexities of clinical research and capitalize on new opportunities in the medical device market.
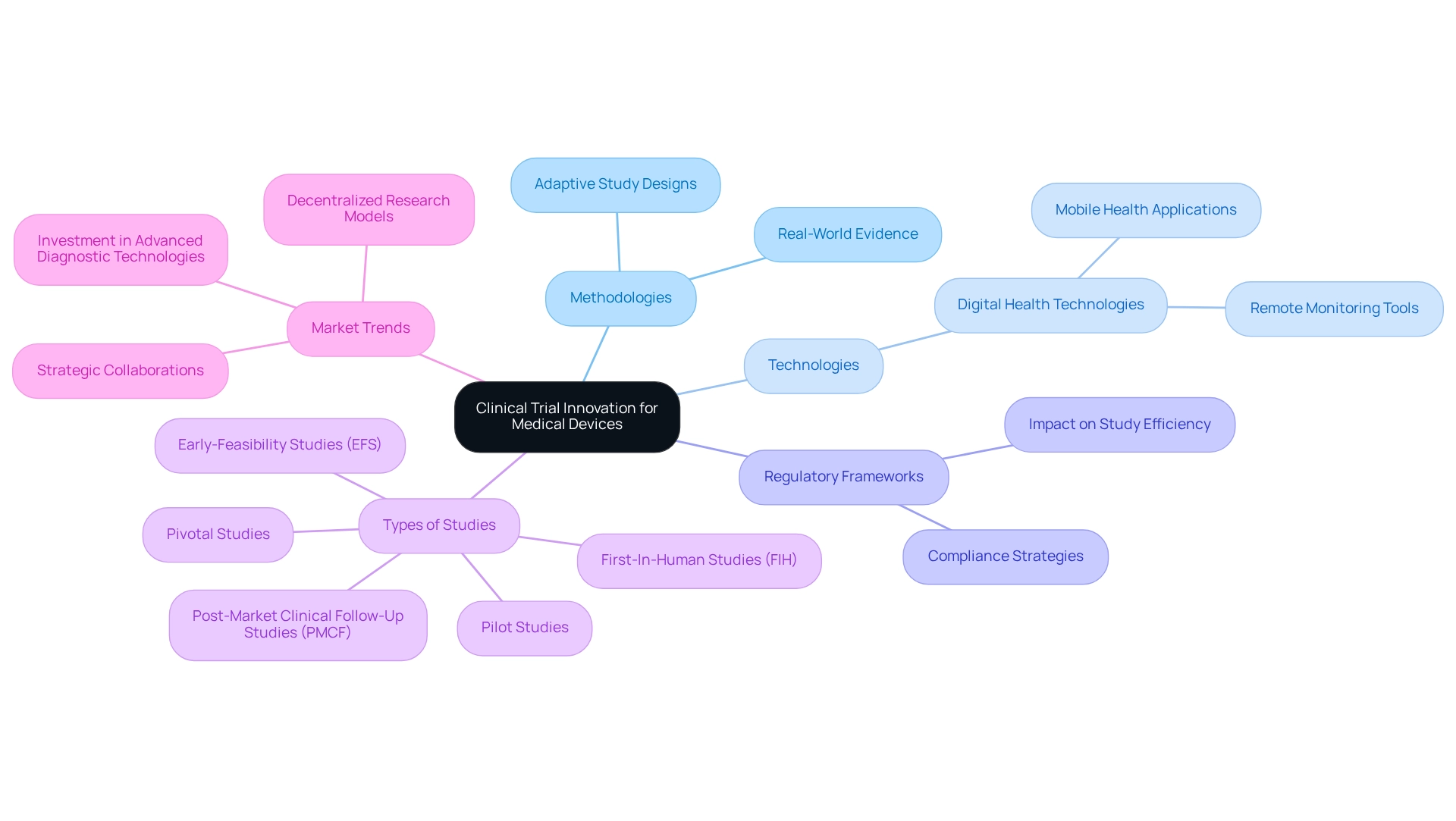
Defining Clinical Trial Innovation for Medical Devices
Clinical trial innovation for devices in Argentina is characterized by the development and implementation of sophisticated methodologies, technologies, and regulatory frameworks designed to enhance the efficiency, effectiveness, and safety of medical device testing. Among the key innovations are adaptive study designs, which allow for modifications to study protocols based on interim results, thereby increasing flexibility and responsiveness to emerging data. The integration of real-world evidence further supports these initiatives, providing insights that facilitate more informed decision-making.
Digital health technologies are pivotal in optimizing research processes, significantly improving patient engagement and data collection. The use of mobile health applications and remote monitoring tools has proven to enhance study efficiency by enabling real-time data access and reducing the burden on patients. By 2025, approximately 10% of the 46,809 registered studies will focus on health technologies, underscoring the growing importance of innovative methodologies in this sector.
At bioaccess®, our commitment to excellence in health technology research design ensures that your studies are not only efficient but also compliant and impactful. With over 20 years of experience in Medtech, we excel in managing a variety of studies, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
This expertise is essential for guaranteeing that processes are both efficient and impactful.
Companies are increasingly investing in advanced diagnostic technologies and decentralized research models to meet the rising demand for specialized services in the healthcare equipment testing market. These strategic shifts, including partnerships among key stakeholders, are shaping a competitive landscape that prioritizes innovation and adaptability.
In conclusion, the strategies and technologies driving clinical trial innovation for devices in Argentina are crucial for accelerating development timelines while ensuring regulatory compliance. By focusing on patient-centered approaches and leveraging advancements in data analytics, the Medtech sector can navigate the complexities of research more effectively, ultimately leading to improved patient outcomes. Furthermore, comprehensive research into the efficacy and limitations of digital tools, along with current applications of AI in healthcare, is vital for enhancing the overall effectiveness of medical experiments.
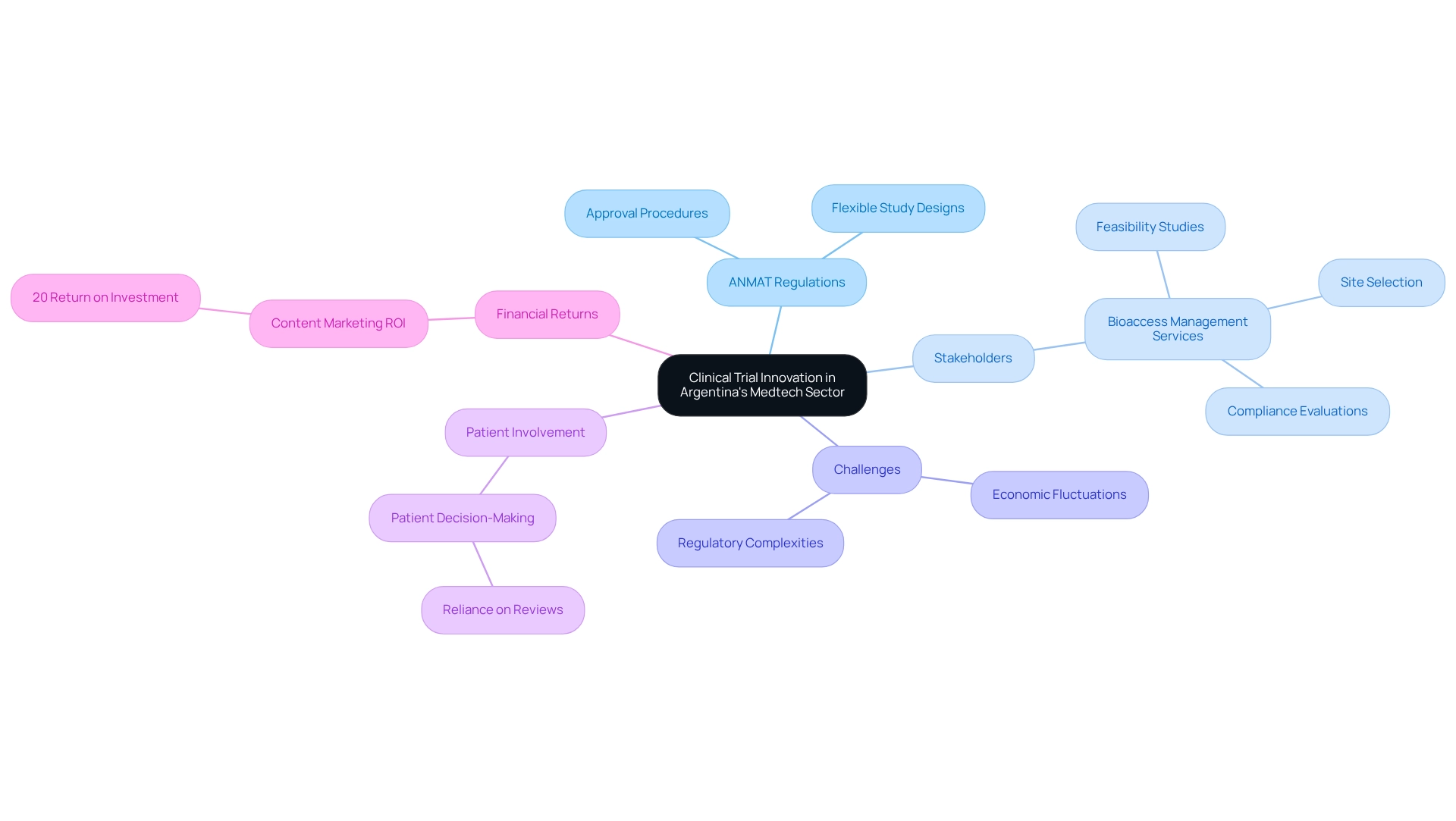
Contextualizing Clinical Trial Innovation in Argentina's Medtech Sector
Argentina's Medtech industry is experiencing a notable surge in demand for innovative medical products, primarily driven by an increasing emphasis on healthcare quality and accessibility. The National Administration of Drugs, Food and Medical Technology (ANMAT) plays a pivotal role in this landscape by modifying its regulatory framework to promote clinical trial innovation for devices in Argentina. Recent updates have streamlined approval procedures and encouraged flexible study designs, which facilitate clinical trial innovation and enable companies to introduce new devices to the market.
The nation's diverse patient demographic, coupled with a robust research framework, positions Argentina as an attractive hub for studies. Statistics reveal that a significant segment of the population actively participates in healthcare decisions; a Stanford study indicates that 75% of patients rely on reviews as their first step in selecting a new doctor or healthcare provider. This highlights the critical importance of patient involvement in the realm of medical studies.
Moreover, investing in research studies and promotion within the Medtech industry can yield substantial financial returns, as evidenced by the statistic that medtech content marketing generates at least a 20% return on investment.
However, the sector faces challenges, including economic fluctuations and regulatory complexities, which can hinder the pace of innovation. To effectively navigate this evolving landscape, stakeholders must stay informed about the latest trends and regulatory updates. For instance, bioaccess® offers a comprehensive range of management services for research studies, encompassing feasibility studies, site selection, compliance evaluations, study setup, import permits, project management, and reporting on both serious and non-serious adverse events.
This expertise is crucial for addressing the regulatory challenges and competition that healthcare product startups often encounter. Furthermore, ANMAT's commitment to fostering research innovation is evident in its recent initiatives aimed at enhancing the efficiency of the approval process. Understanding these dynamics is essential for Medtech firms looking to engage in clinical trial innovation for devices in Argentina, which can lead to improved health-related devices and support local economic development through job creation and enhanced healthcare outcomes.
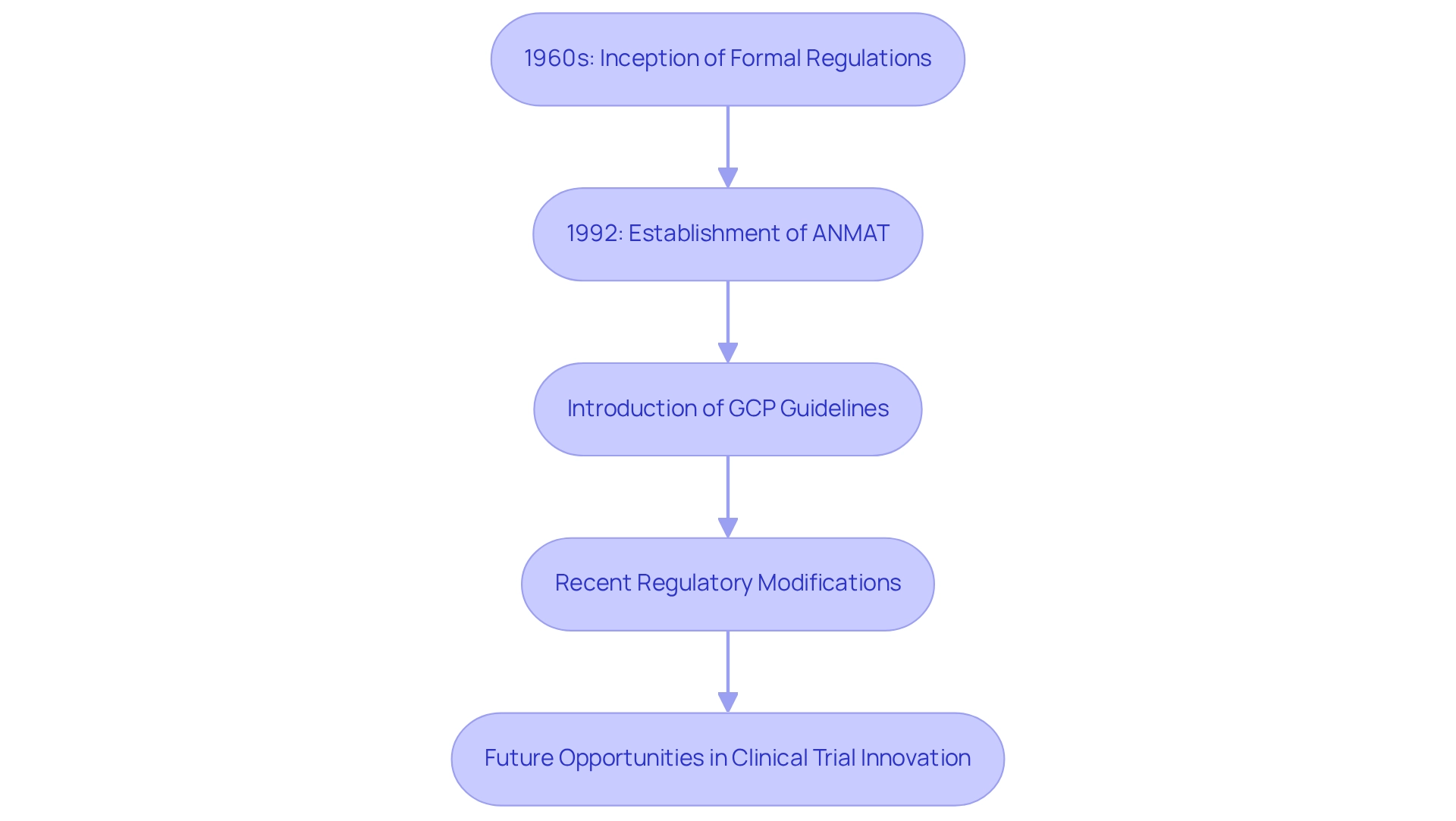
Tracing the Evolution of Clinical Trials in Argentina
The evolution of medical studies in Argentina can be traced back to the 1960s, marking the inception of formal regulations for health research. Over the decades, this landscape has undergone significant transformation, particularly with the establishment of the National Administration of Drugs, Food and Medical Technology (ANMAT) in 1992. This pivotal development centralized regulatory oversight, ensuring that studies adhere to rigorous standards.
The introduction of Good Clinical Practice (GCP) guidelines has further aligned Argentina's regulations with international benchmarks, thereby enhancing the credibility of its research. In recent years, a collective initiative has emerged to modernize the research framework. Regulatory modifications have focused on expediting approval processes and promoting innovative study designs, which are crucial for fostering clinical trial innovation for devices in Argentina. For instance, a case study evaluating the integration of the International Committee of Medical Journal Editors (ICMJE) trial registration policy revealed that numerous biomedical journals in Argentina still face challenges with adherence, underscoring the necessity for ongoing education and advocacy for trial registration among stakeholders.
This aligns with the priorities outlined by Fernando Daniel Gassin, Solutions Architect at the Argentine Ministry of Health, who stated, "This year, our focus is to keep enhancing the interoperability of our regional healthcare systems with the Red Hat team."
Moreover, it is essential to recognize that the FDA is regarded as the most advanced agency among reference health authorities concerning regulatory updates, serving as a benchmark for Argentina's regulatory framework. This historical perspective illustrates Argentina's commitment to improving research practices, particularly through clinical trial innovation for devices, thereby positioning the nation as a competitive player in the global Medtech industry. As the regulatory landscape continues to evolve, it presents exciting opportunities for groundbreaking health technology firms, such as bioaccess®, to engage in clinical trial innovation for devices in Argentina, potentially leading to significant advancements in healthcare.
Furthermore, bioaccess® is dedicated to ensuring information security and client trust throughout the clinical study process. Our commitment to data protection is evident in our grievance procedures, which empower clients to address any concerns regarding the handling of their information. Clients can contact our Grievance Officer at IMH ASSETS CORP (doing business as "bioaccess®") for any inquiries, ensuring compliance and transparency in all our operations.
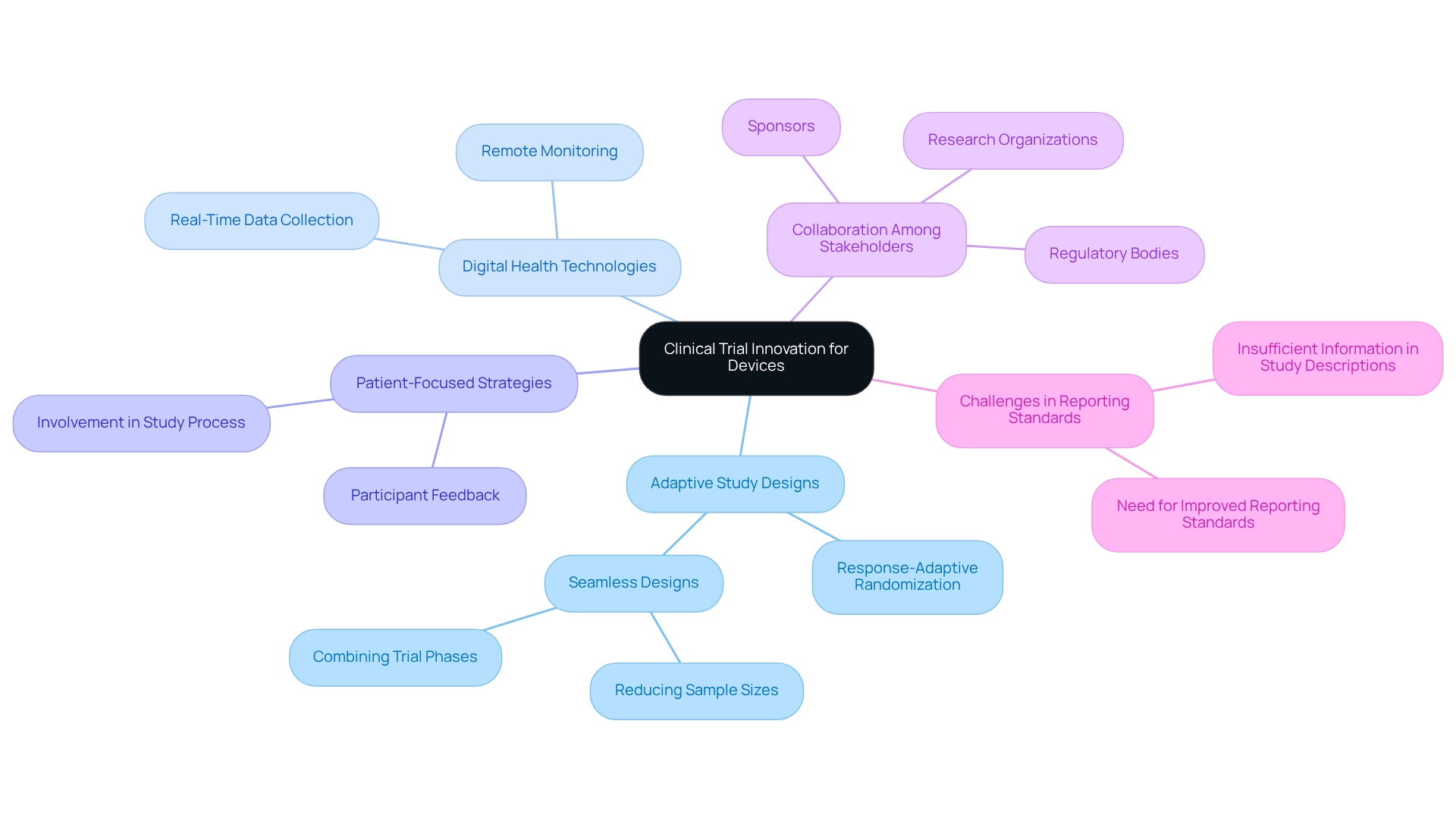
Key Characteristics of Clinical Trial Innovation for Devices
Clinical trial innovation for devices in Argentina prominently features several key components that enhance both efficiency and quality. At the forefront are adaptive study designs, which enable modifications based on interim results, significantly streamlining the development process. The ICECAP study exemplifies this by utilizing response-adaptive randomization to optimize hypothermia duration for neuroprotection in cardiac arrest survivors, illustrating how adaptive designs can lead to more effective treatment protocols.
Another critical aspect is the incorporation of digital health technologies, which facilitate remote monitoring and real-time data collection. This integration not only boosts participant engagement but also enhances data accuracy, thereby simplifying the assessment of device performance across diverse populations. Recent statistics reveal that only 3.1% of studies are in phase IV, highlighting the pressing need for clinical trial innovation for devices in Argentina to accelerate the transition from early-stage research to market.
Patient-focused strategies are increasingly prioritized, emphasizing participant feedback and involvement throughout the study process. This shift is vital for ensuring that trials align with patient needs and expectations. Moreover, the integration of real-world evidence alongside conventional medical data is becoming a hallmark of innovation, enabling researchers to evaluate device efficacy in broader, more representative populations.
Collaboration among stakeholders—including regulatory bodies, sponsors, and research organizations—is essential for creating an environment conducive to clinical trial innovation for devices in Argentina. Bioaccess®, with over 20 years of expertise in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), exemplifies a commitment to regulatory excellence and innovation. The challenges faced in recognizing adaptive designs, as underscored by recent case studies, highlight the necessity for enhanced reporting standards in research studies.
Specifically, the case study titled "Challenges in Identifying Adaptive Designs" illustrates that insufficient information in study descriptions complicates the identification of adaptive designs, underscoring the need for clearer reporting.
Furthermore, seamless designs that integrate trial phases have the potential to expedite development timelines and reduce sample sizes, further enhancing research trial efficiency. As the landscape of clinical research evolves, these characteristics collectively contribute to faster access to new medical devices, ultimately benefiting patients and advancing healthcare outcomes.
Conclusion
The landscape of clinical trials for medical devices is experiencing a profound transformation, propelled by innovative methodologies that significantly enhance efficiency and patient outcomes. Key advancements, including adaptive trial designs and the integration of digital health technologies, are leading this evolution. These innovations facilitate real-time modifications based on interim results and enhance patient engagement through improved data collection methods. The increasing percentage of registered studies focusing on medical devices underscores the growing importance of these methodologies, reflecting a shift toward more responsive and patient-centric clinical research.
In the context of Argentina's burgeoning Medtech sector, the commitment of regulatory bodies like ANMAT to streamline approval processes and adopt adaptive designs further solidifies the country's position as a competitive hub for clinical trials. The diverse patient population and robust clinical research infrastructure in Argentina present unique opportunities for stakeholders to leverage these innovations effectively. However, navigating challenges such as economic fluctuations and regulatory complexities is essential to maximize the potential benefits of clinical trial investments.
Overall, the innovations shaping clinical trials for medical devices are crucial not only for accelerating development timelines but also for ensuring that trials remain compliant and impactful. By embracing these advancements, the Medtech industry can adeptly navigate the complexities of clinical research, ultimately leading to improved healthcare outcomes. As the sector continues to evolve, it is imperative for stakeholders to stay informed about the latest trends and regulatory updates to capitalize on new opportunities in this dynamic market.
Frequently Asked Questions
What characterizes clinical trial innovation for devices in Argentina?
Clinical trial innovation for devices in Argentina is characterized by the development and implementation of sophisticated methodologies, technologies, and regulatory frameworks aimed at enhancing the efficiency, effectiveness, and safety of medical device testing.
What are adaptive study designs and why are they important?
Adaptive study designs allow for modifications to study protocols based on interim results, increasing flexibility and responsiveness to emerging data, which is crucial for improving trial outcomes.
How does real-world evidence contribute to clinical trials?
The integration of real-world evidence provides insights that facilitate more informed decision-making, supporting the overall objectives of clinical trial innovations.
What role do digital health technologies play in clinical trials?
Digital health technologies optimize research processes by improving patient engagement and data collection, enhancing study efficiency through real-time data access and reducing the burden on patients.
What is the projected focus of registered studies by 2025?
By 2025, approximately 10% of the 46,809 registered studies will focus on health technologies, highlighting the growing importance of innovative methodologies in this sector.
What types of studies does bioaccess® manage?
Bioaccess® manages a variety of studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).
What trends are influencing the healthcare equipment testing market?
Companies are increasingly investing in advanced diagnostic technologies and decentralized research models, along with forming partnerships among key stakeholders, which are shaping a competitive landscape that prioritizes innovation and adaptability.
Why are patient-centered approaches and data analytics important in Medtech?
Focusing on patient-centered approaches and leveraging advancements in data analytics allows the Medtech sector to navigate the complexities of research more effectively, ultimately leading to improved patient outcomes.
What is the significance of researching digital tools in clinical trials?
Comprehensive research into the efficacy and limitations of digital tools, along with current applications of AI in healthcare, is vital for enhancing the overall effectiveness of medical experiments.




