Overview
Understanding design control for medical devices involves a structured approach that ensures compliance with regulatory standards and enhances product quality throughout the development lifecycle. The article outlines key phases of the design control process, emphasizing the importance of thorough documentation, rigorous verification, and validation activities, which collectively help mitigate risks and improve patient safety in alignment with regulatory requirements.
Introduction
In the highly regulated medical device industry, the importance of design control cannot be overstated. This structured approach is pivotal in guiding the development and manufacturing processes, ensuring that devices not only meet user requirements but also adhere to stringent regulatory standards.
As the landscape evolves, organizations are increasingly recognizing the critical role of comprehensive design control in mitigating risks, enhancing product quality, and fostering compliance with authorities such as INVIMA. This article delves into the fundamental components of design control, the key phases of the process, and the regulatory frameworks that shape its implementation.
Additionally, it explores the myriad benefits of effective design control and addresses common misconceptions that can hinder progress in this vital area. By understanding and embracing these principles, stakeholders can contribute to the safety and efficacy of medical devices, ultimately improving patient outcomes and advancing the industry as a whole.
Fundamentals of Design Control in Medical Devices
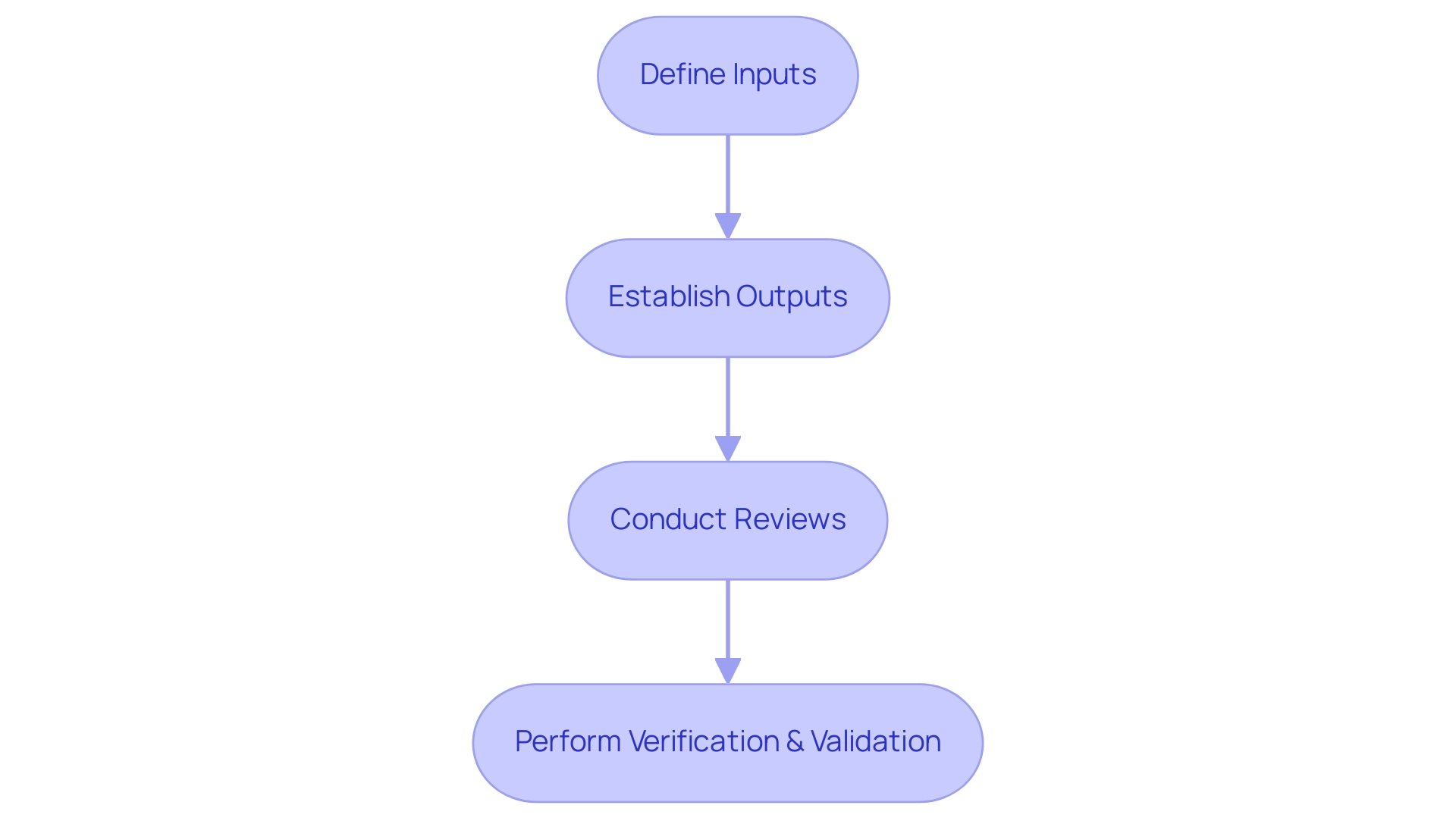
Design control medical device training focuses on the structured and methodical approach that is essential for controlling the creation process of medical devices, ensuring that they meet both user requirements and regulatory standards. This procedure encompasses a series of coordinated activities critical throughout the product lifecycle. Fundamental components include:
- Defining clear inputs
- Establishing measurable outputs
- Conducting thorough reviews
- Performing rigorous verification and validation activities
By implementing design control medical device training, organizations can substantially lower the likelihood of product failures, ultimately enhancing their overall quality management systems. This is particularly crucial in light of recent statistics indicating that recalls recommending the discontinuation of affected products were terminated 31.8% of the time, compared to only 18.2% for those suggesting assessment and/or education. Furthermore, it is advised to avoid signing any authorization forms to release medical records if impacted by a medical recall, as manufacturers may misuse this access.
As mentioned by Arkeliana Tase, 'Enhanced communication strategies should be established between healthcare and the MedTech sector,' emphasizing the necessity for partnership to improve development procedures. Moreover, a recent study on surgical tools emphasizes the importance of these processes and suggests that further research involving diverse clinician groups and manufacturers is needed to enhance understanding and improve reporting and safety. The implementation of effective design control medical device training not only safeguards patient safety but also ensures compliance with evolving regulatory landscapes governed by authorities like INVIMA, the Colombia National Food and Drug Surveillance Institute, recognized as a Level 4 health authority by the PAHO/WHO.
Within INVIMA, the Directorate for Medical Equipment and other Technologies plays a critical role in monitoring and controlling medical instruments, ensuring adherence to technical standards throughout the pre-market and post-market phases. This emphasizes the significance of these methods in the medical equipment sector, ensuring compliance with the highest regulatory standards and reducing the risk of breaches of health standards or procedures.
Key Phases of the Design Control Process
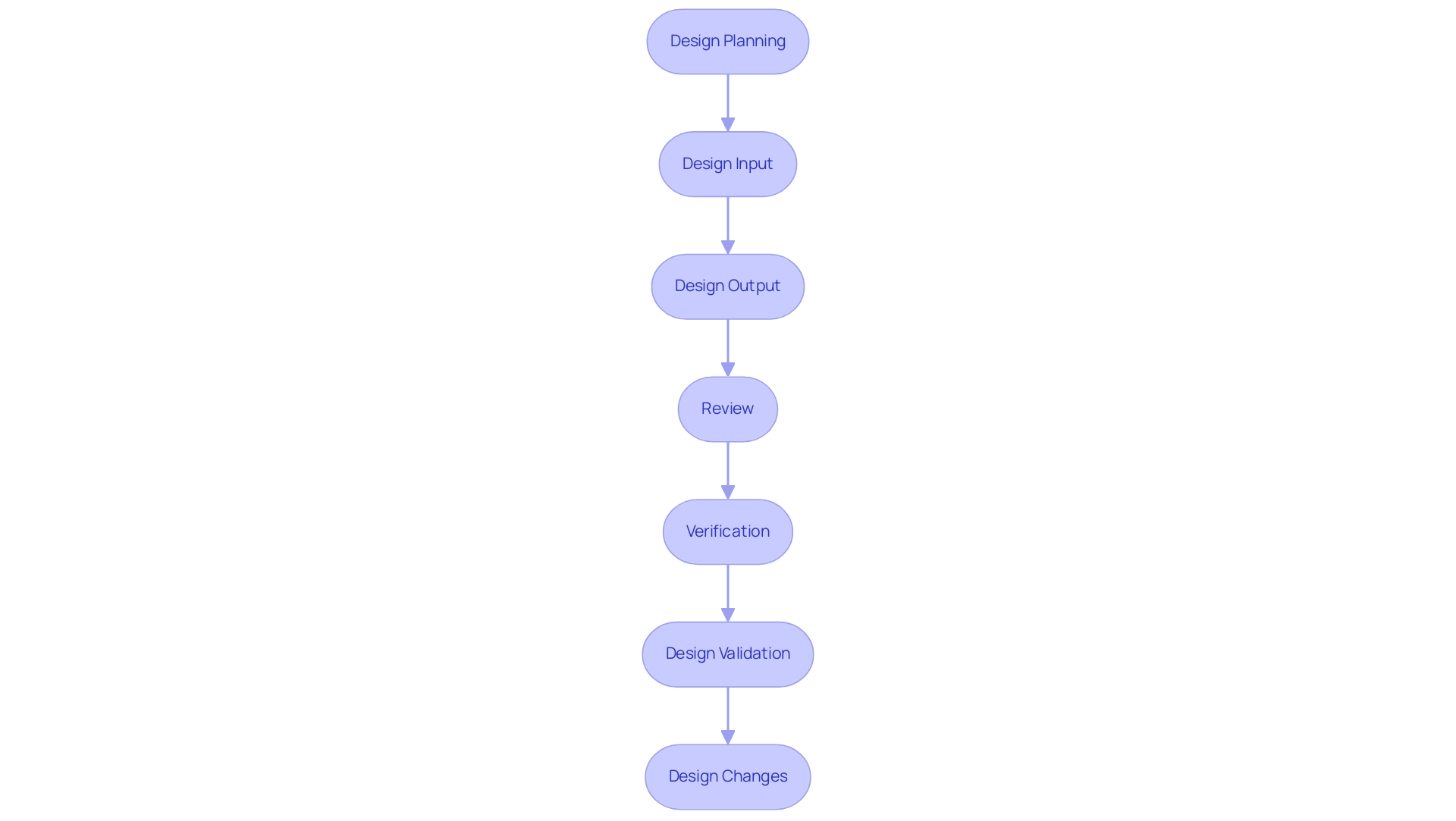
The control framework for medical products is a systematic method that includes several essential stages, each fulfilling a unique role in guaranteeing adherence and quality, especially under the supervision of regulatory bodies such as INVIMA. The process begins with Design Planning, where clear objectives are outlined, and specific tasks are defined to guide the development. Following this, Design Input concentrates on collecting user requirements, regulatory standards, and defining the intended use and indications for the product, including considerations for the patient population, which must align with INVIMA regulations.
Next, Design Output entails documenting specifications and features that comply with the established requirements. This is succeeded by the Review phase, where evaluations are conducted at various stages to confirm alignment with the defined requirements and objectives.
Verification is a crucial step that ensures the outputs meet the specified inputs, confirming that all user needs are addressed, in compliance with INVIMA's stringent standards. Afterward, Design Validation ensures that the final product satisfies the intended purposes and aligns with the expectations of the end-users, an essential factor considering INVIMA's responsibility in supervising medical device effectiveness and safety.
Ultimately, Design Changes must be managed through a systematic approach that records any alterations made, both pre and post-release, to uphold compliance with regulatory requirements. As emphasized by the FDA,
You can have volumes of procedures to show to an auditor, but if you can’t show that they’ve been implemented you're not in compliance.
Organizations must record their planning for creation and development, including design control medical device training, to formulate plans with stages that correspond with key milestones in the development phase.
An example of effective documentation is the History File (DHF), which must comprehensively record all creation activities, ensuring a thorough account of the development stages and facilitating future audits. A well-maintained DHF provides a comprehensive record of the creation phase, ensuring compliance with regulatory requirements, particularly those set forth by INVIMA, and facilitating future audits, as shown in the case study titled 'Completing Changes and the Design History File (DHF)'. Moreover, clients can access a network of over 4000 compliance specialists globally, boosting the credibility of the development framework and offering valuable resources to navigate regulatory challenges, especially concerning INVIMA's guidelines.
By following these organized stages, organizations can manage the challenges of development while ensuring their products meet strict regulatory criteria and user expectations. INVIMA's designation as a Level 4 health authority highlights its proficiency in overseeing medical products, ensuring that all procedures conform to global benchmarks for safety, efficacy, and quality.
Regulatory Framework and Compliance for Design Controls
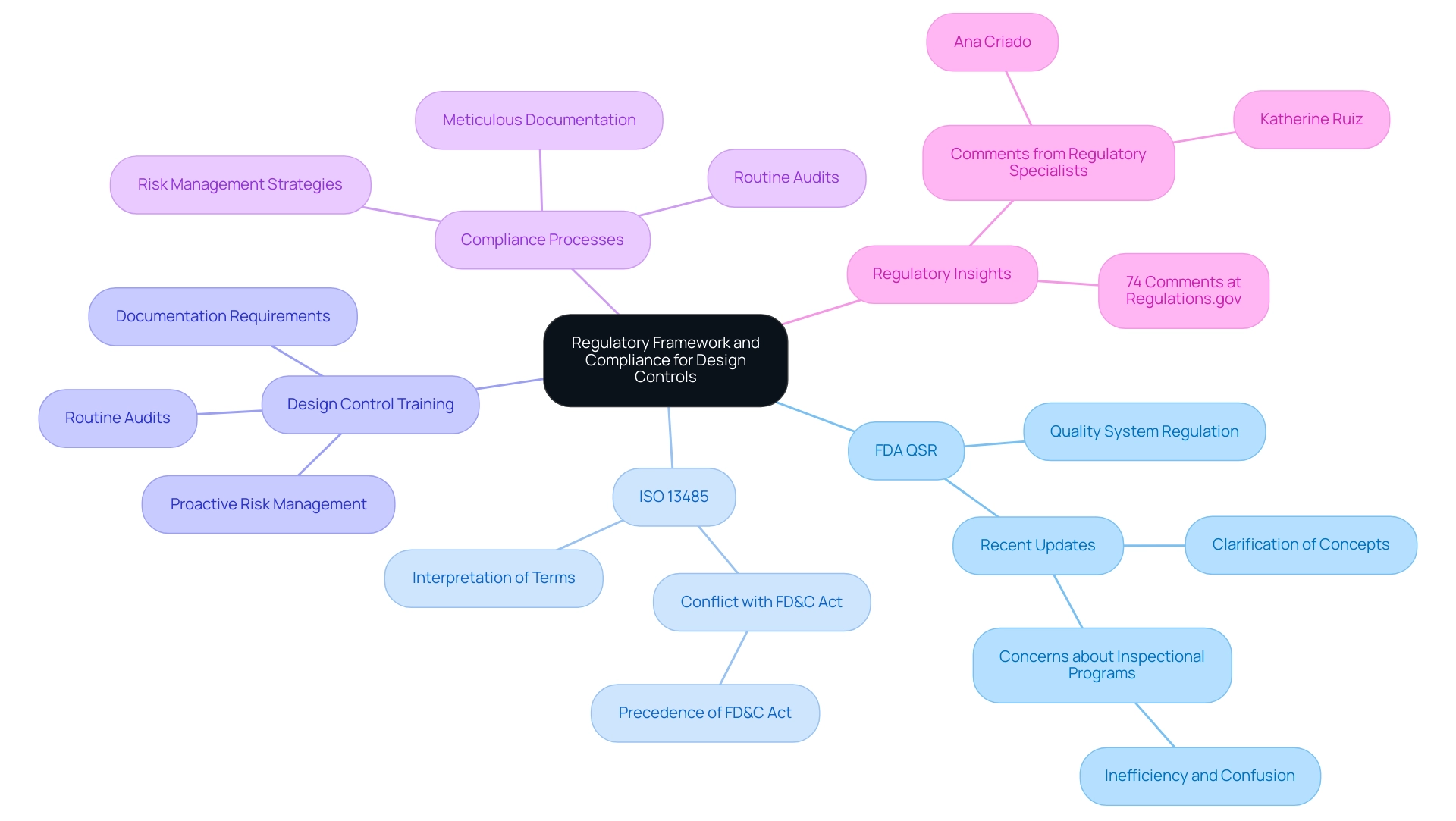
The regulatory environment overseeing product standards in medical equipment manufacturing is fundamentally influenced by the FDA's Quality System Regulation (QSR) and ISO 13485 standards. These regulations necessitate manufacturers to implement and maintain design control medical device training throughout the complete product lifecycle, a procedure that is vital to guaranteeing product safety and effectiveness. Compliance in design control medical device training entails meticulous documentation, routine audits, and proactive risk management to effectively meet the stipulated standards.
Recent updates highlight the FDA's commitment to enhancing clarity within these regulations, as seen in their decision to clarify concepts from §820.15 to §820.3(b), improving readability and interpretation of the QMSR. Furthermore, the FDA has expressed concerns about having two inspectional programs in operation simultaneously, stating, 'FDA disagrees that a phased-in effective date is appropriate, because having two inspectional programs in operation at the same time would be inefficient and would result in significant potential for confusion.' This underscores the importance of a streamlined approach to compliance.
Manufacturers must also interpret terms outlined in ISO 13485 in alignment with definitions provided by the FDA, which is vital for design control medical device training and navigating regulatory requirements. For instance, understanding how the definitions of 'design validation' differ between ISO 13485 and the FD&C Act can impact compliance strategies. The intricacies of this regulatory framework, as highlighted by regulatory specialists such as Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, and Katherine Ruiz, an authority in Regulatory Affairs for medical products and in vitro diagnostics in Colombia, are further emphasized by the fact that 74 comments have been received at Regulations.gov, reflecting ongoing discussions and considerations within the field.
Furthermore, a recent case study emphasized the conflict between ISO 13485 clauses and provisions of the FD&C Act, illustrating that in instances of disagreement, the FD&C Act and its regulations will take precedence, highlighting the essential need for manufacturers to remain aware of these regulatory nuances.
Benefits of Effective Design Control Implementation
The efficient execution of specifications in design control medical device training and production results in numerous important advantages, which consist of:
-
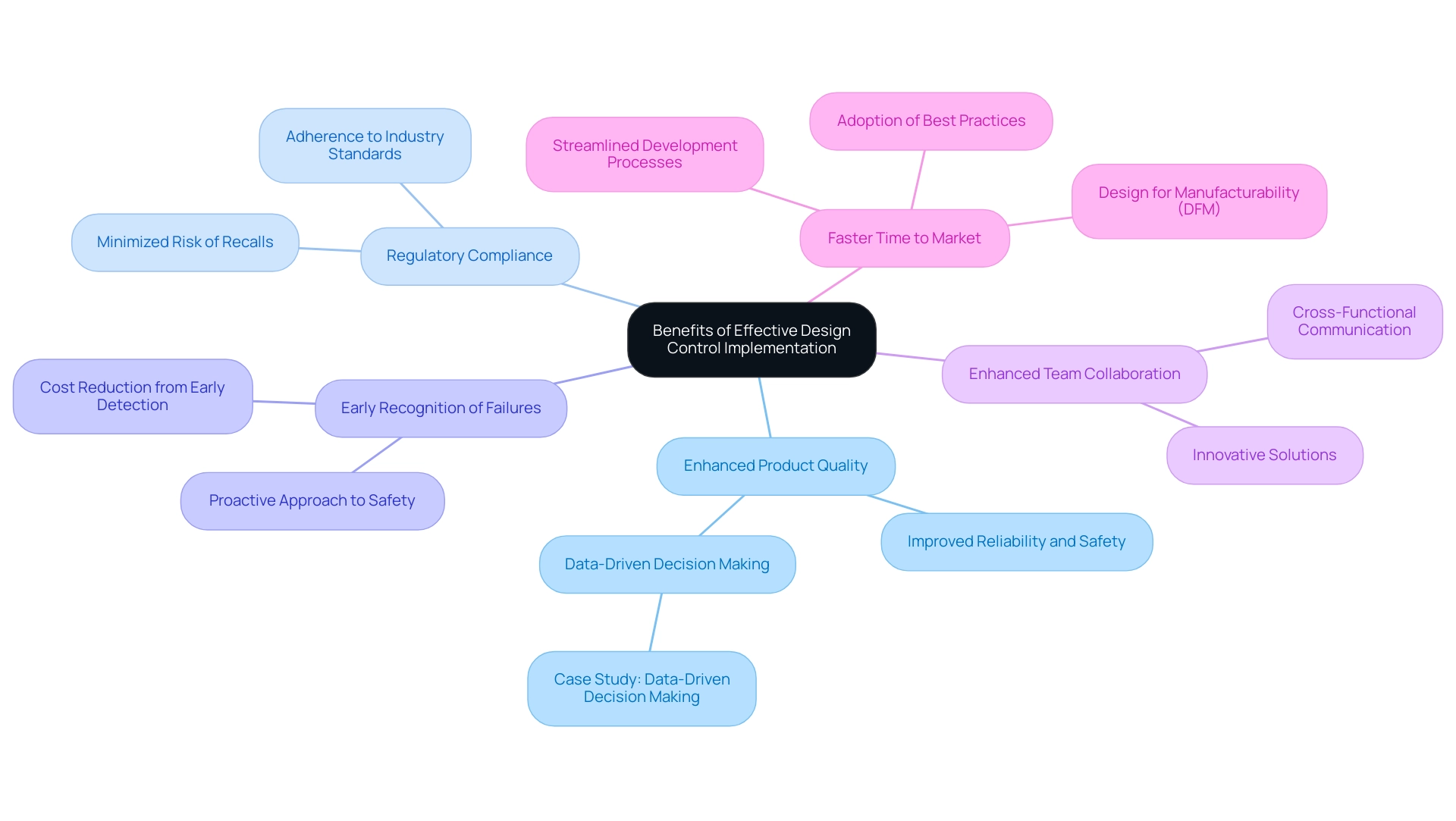
Enhanced Product Quality: Fundamental to the design control medical device training process is the improvement of reliability and safety, ensuring that medical devices consistently meet both user and regulatory expectations. Recent studies show that organizations using strict development regulations have recorded significant advancements in product quality, resulting in improved patient outcomes.
For instance, the case study titled 'Data-Driven Decision Making' illustrates how utilizing data analysis can guide product engineering decisions, ultimately enhancing product quality and reducing time-to-market.
-
Regulatory Compliance: Following established industry standards is essential, and effective design control medical device training measures aid in meeting these standards, thus minimizing the risk of product recalls and related legal consequences.
-
Through design control medical device training, organizations can recognize potential structural failures early in the development phase, which helps to reduce expensive mistakes and improve overall product safety. This proactive approach not only protects consumers but also safeguards the organization’s reputation.
-
Enhanced Team Collaboration: Design control medical device training measures promote communication among cross-functional teams, fostering an organizational culture that prioritizes quality. This collaborative environment leads to innovative solutions and more effective problem-solving.
-
Faster Time to Market: Streamlined development processes that include design control medical device training enable organizations to respond more efficiently to market demands, significantly reducing timelines for development. Organizations that adopt best practices and leverage advanced technologies can notably improve their product development outcomes by implementing design control medical device training, optimizing both time and costs.
As Waqar Ahmad Malik aptly stated, 'It’s that magical time when we bid farewell to the past year and welcome a fresh start,' emphasizing the continuous improvement mindset necessary in the evolving landscape of medical device development. In the context of 2024, the advantages of management are increasingly acknowledged, especially as the sector readies for forthcoming challenges and opportunities. Moreover, the application of Design for Manufacturability (DFM) is suggested to lower expenses and enhance product quality by enabling faster assembly from fewer components and promoting standardization, further aligning with the advantages of efficient management.
Navigating Challenges and Misconceptions in Design Control
The framework of regulatory oversight for medical products presents several challenges, including insufficient documentation, opposition to change, and a restricted understanding of the extent of regulatory measures. Misunderstandings often arise in the industry, particularly the notion that development regulations apply only to high-risk products or can be disregarded during accelerated development processes. According to the FDA, incorporating lessons from previous verification and validation efforts can improve future product constructions.
This highlights the significance of appropriate management across all product classifications. Notably, ISO 13485:2016 7.3 outlines the input requirements for medical devices, providing a regulatory framework that emphasizes the necessity of thorough documentation. The Device Master Record (DMR) and the Design History File (DHF) are essential elements that record the specifications and development stages, ensuring clarity and compliance throughout the oversight.
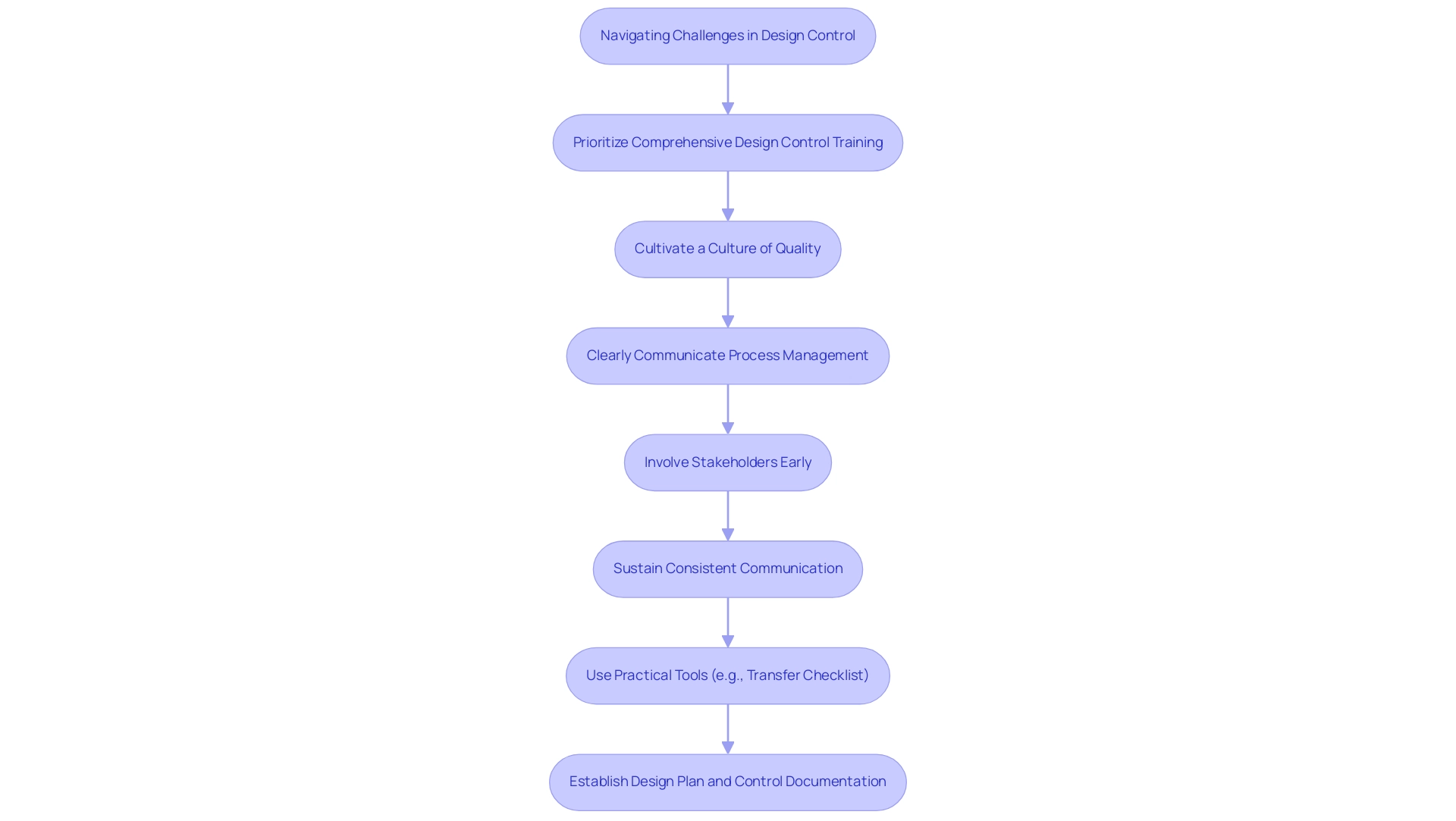
To effectively navigate these hurdles, it is essential for professionals to:
- Prioritize comprehensive design control medical device training
- Cultivate a culture that values quality
- Ensure that process management is communicated clearly across all teams
Involving stakeholders early and sustaining consistent communication can help tackle concerns and align expectations, thereby promoting a more resilient oversight environment. Furthermore, using practical tools like a transfer checklist can help in ensuring that all necessary steps are meticulously followed.
Furthermore, establishing a design plan and control documentation, as seen in the case study on Documenting Design and Development Planning, ensures that all necessary steps are meticulously followed, with well-defined team roles and adherence to established plans.
Conclusion
Implementing a robust design control process is not merely a regulatory requirement; it is a fundamental pillar that significantly enhances the quality and safety of medical devices. By adhering to the structured phases of design control—from planning and input gathering to output documentation and validation—organizations can ensure that their products not only meet user needs but also comply with stringent regulatory standards. This meticulous approach is essential in mitigating risks, preventing costly recalls, and fostering a culture of quality and collaboration within teams.
The regulatory frameworks, including the FDA's Quality System Regulation and ISO 13485 standards, provide a comprehensive structure that guides manufacturers in maintaining compliance throughout the product lifecycle. Understanding these regulations and their implications is crucial for navigating the complexities of the medical device landscape. Furthermore, dispelling common misconceptions about design controls is vital in promoting their widespread adoption across all device classifications, ensuring that every product benefits from thorough oversight.
Ultimately, the advantages of effective design control implementation are far-reaching, leading to improved product quality, enhanced patient safety, and a faster time to market. As organizations continue to evolve in this dynamic industry, embracing design control principles will be pivotal in driving innovation, ensuring compliance, and ultimately advancing the field of medical devices for the benefit of patients and healthcare providers alike. Prioritizing these practices is essential for a successful future in medical device development, underscoring the critical role design control plays in safeguarding health outcomes.
Frequently Asked Questions
What is the purpose of design control medical device training?
Design control medical device training focuses on ensuring a structured and methodical approach to the creation process of medical devices, helping them meet user requirements and regulatory standards throughout the product lifecycle.
What are the fundamental components of design control in medical device training?
The fundamental components include defining clear inputs, establishing measurable outputs, conducting thorough reviews, and performing rigorous verification and validation activities.
How does design control training impact product quality?
Implementing design control medical device training can significantly lower the likelihood of product failures, thereby enhancing overall quality management systems.
What recent statistics highlight the importance of design control in medical devices?
Recent statistics indicate that recalls recommending the discontinuation of affected products were terminated 31.8% of the time, compared to only 18.2% for those suggesting assessment and/or education.
What should individuals do if they are impacted by a medical recall?
It is advised to avoid signing any authorization forms to release medical records, as manufacturers may misuse this access.
What role does INVIMA play in medical device regulation?
INVIMA, the Colombia National Food and Drug Surveillance Institute, monitors and controls medical instruments to ensure compliance with technical standards throughout the pre-market and post-market phases.
What are the key stages in the control framework for medical products?
The key stages include Design Planning, Design Input, Design Output, Review, Verification, Design Validation, and Design Changes.
What is the significance of the Design History File (DHF)?
The DHF is a comprehensive record of all creation activities, ensuring compliance with regulatory requirements and facilitating future audits.
How does the FDA view documentation in compliance?
The FDA emphasizes that having procedures is not enough; organizations must demonstrate that these procedures have been implemented to be considered compliant.
What resources are available to organizations navigating regulatory challenges?
Organizations can access a network of over 4000 compliance specialists globally, which can enhance credibility and provide valuable resources related to regulatory challenges, especially concerning INVIMA's guidelines.

