Overview
Pilot studies can significantly accelerate medical device approval by assessing feasibility and identifying challenges early in the development process, ultimately leading to improved health outcomes and streamlined regulatory pathways. The article emphasizes that these preliminary investigations not only enhance device designs and safety data but also provide vital insights for regulatory bodies, thus facilitating a more efficient approval process for innovative medical technologies.
Introduction
In the intricate landscape of medical device development, pilot studies emerge as a pivotal component in the journey toward regulatory approval. These preliminary investigations not only evaluate the feasibility and safety of new devices but also provide essential insights that can significantly influence subsequent clinical trials.
By identifying potential challenges early on, pilot studies facilitate the refinement of study designs and enhance the likelihood of successful outcomes. As the healthcare industry increasingly relies on innovative technologies, understanding the multifaceted role of pilot studies becomes crucial for developers and regulatory bodies alike.
This article delves into the various aspects of pilot studies, exploring their:
- Methodologies
- Benefits
- Regulatory considerations
- Challenges they face
Ultimately, it underscores their importance in advancing medical device research and ensuring patient safety.
The Role of Pilot Studies in Medical Device Approval
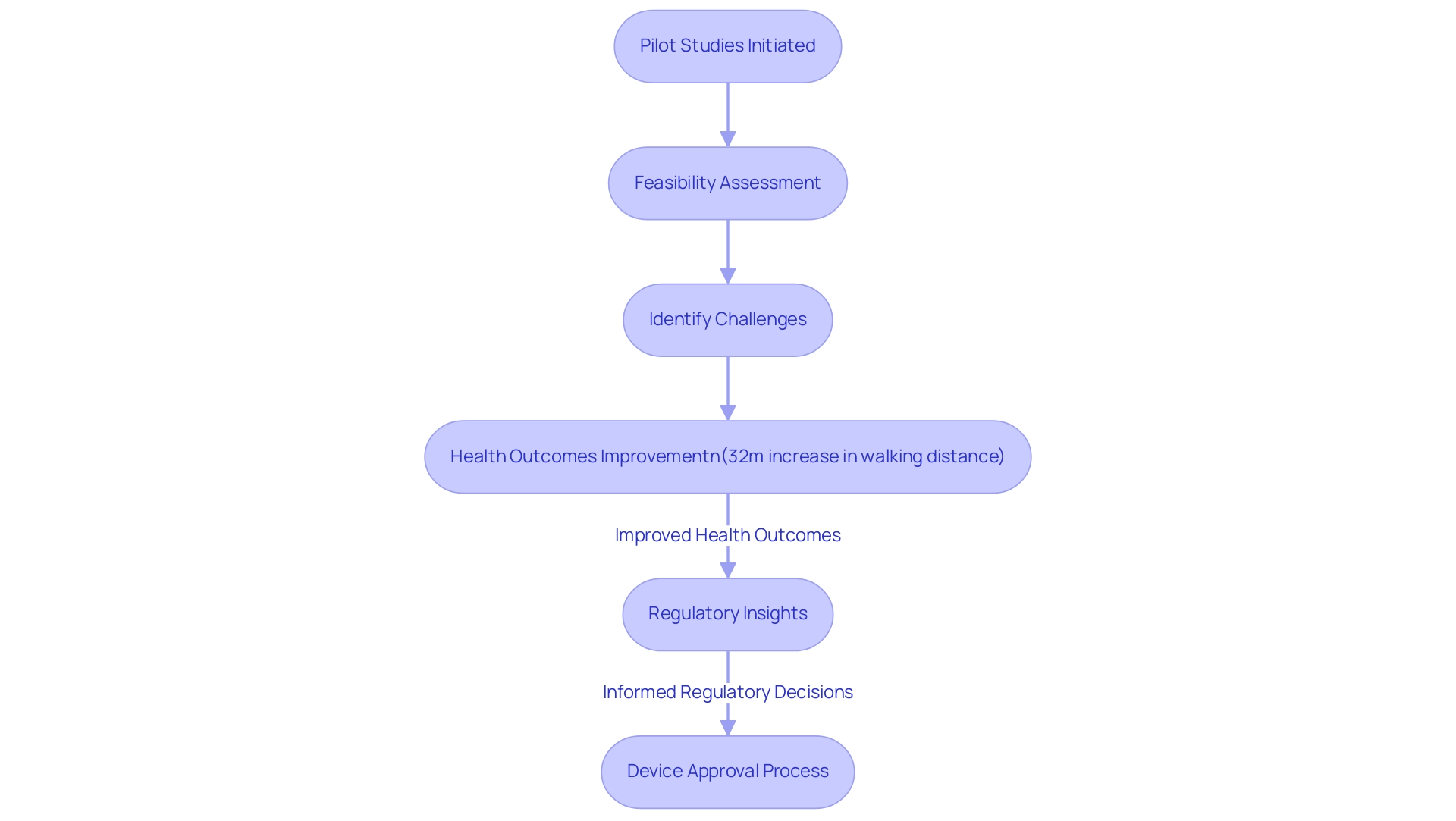
Pilot investigations, frequently called feasibility assessments, function as initial examinations aimed at assessing the viability, duration, expense, and possible negative occurrences linked with larger research trials. Within the realm of medical device approval, it is essential to understand how pilot studies can accelerate medical device approval. They provide developers, such as Avantec Vascular, with the opportunity to assess the practicality of their devices in real-world settings, identifying potential challenges early in the process.
For example, Avantec's first-in-human trial of an innovative vascular device in Latin America, supported by bioaccess™, demonstrates the essential role of preliminary research in enhancing health outcomes. Significantly, preliminary investigations have demonstrated enhancements in health results, as indicated by a notable 32-meter rise in the 6-minute walking distance among participants utilizing specific medical devices. Moreover, preliminary investigations play a vital role in creating dependable techniques for assessing clinical results, especially for subjective endpoints such as pain and quality of life.
This validation process ensures that the assessment of outcomes is accurate and reliable for future research. Tracking compliance with the treatment regimen is also essential for the success of home-based therapies, as it directly influences the effectiveness of the intervention. Furthermore, preliminary investigations provide insights that are essential for oversight organizations during the approval process, as emphasized by specialists such as Katherine Ruiz, who focuses on compliance matters for medical devices in Colombia.
According to Karen Becker Witkin, a Principal with the Weinberg Group, Inc., 'Pilot projects can assist manufacturers not only in creating better devices, but also in saving time, money, and resources.' By tackling these initial questions, preliminary research demonstrates how pilot studies can accelerate medical device approval, streamlining the path to market and ensuring that devices conform to required standards, ultimately improving the success rates of clinical trials. Furthermore, preliminary investigations should concentrate on evaluating the practicality and acceptability of methods for larger research, ensuring they are timely and pertinent in the present context of medical device advancement.
Bioaccess™ facilitates this process by providing essential services such as the selection of a principal investigator and the submission of compliance dossiers, which directly support the approval process. Insights acquired from preliminary experiments not only inform device development but also play a crucial role in shaping regulatory decisions, thereby enhancing the overall efficacy of clinical trials.
Benefits and Methodologies of Conducting Pilot Studies
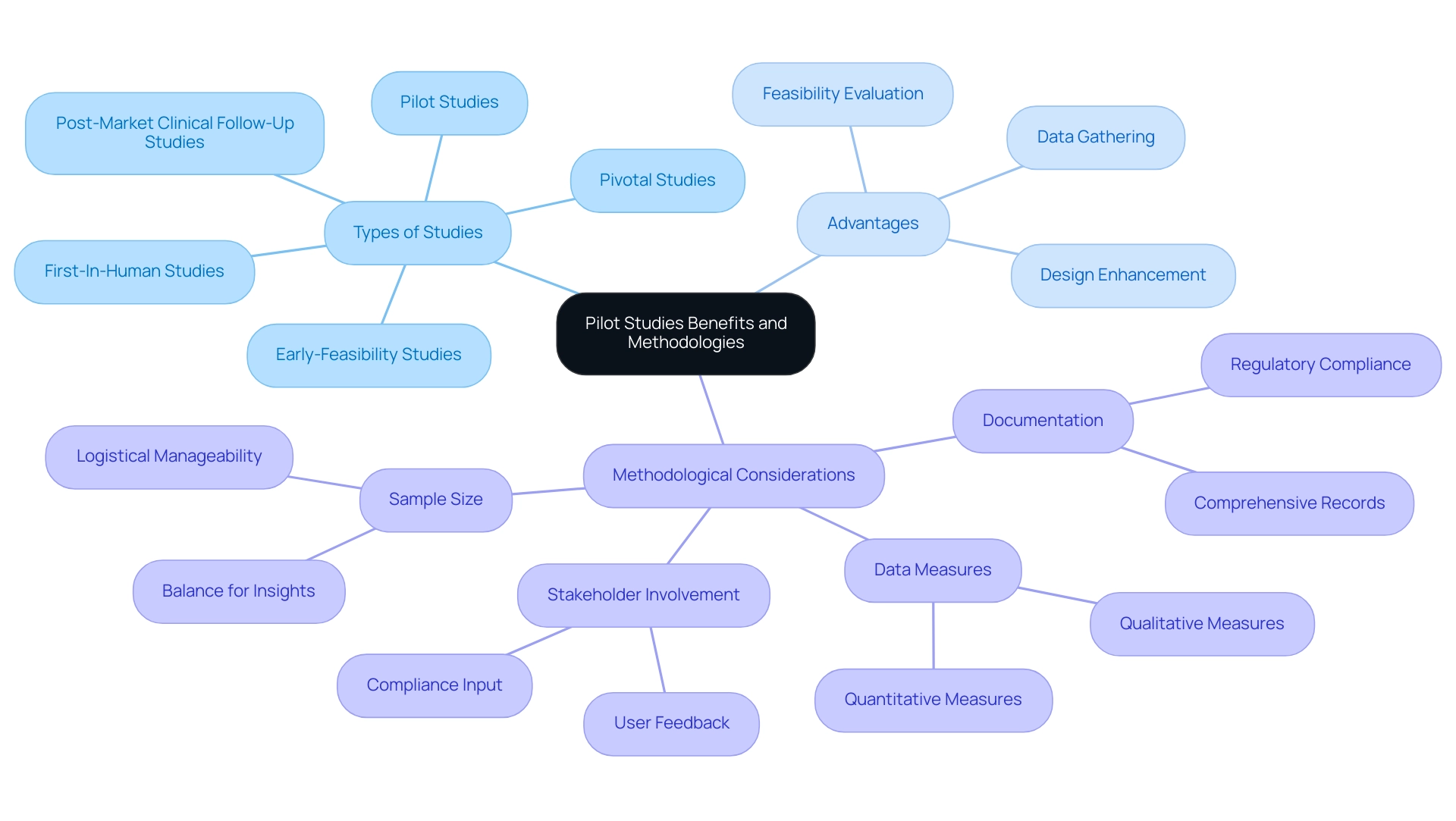
Conducting a pilot investigation presents a myriad of advantages, particularly in understanding how pilot studies can accelerate medical device approval. These investigations provide researchers the chance to evaluate the feasibility of a device, enhance designs, and gather preliminary safety and efficacy data. With the support of bioaccess®, a vetted partner in clinical trials across Latin America, researchers can leverage over 20 years of expertise in managing:
- Early-Feasibility Studies
- First-In-Human Studies
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies
However, there is ongoing discussion about whether preliminary experiments can reliably assess the safety or tolerability of a treatment. Considering that comprehensive accounts on preliminary experiments are limited in the research literature, their significance is heightened. Effective approaches for conducting trial research include choosing a sample size that strikes a balance; it must be sufficiently large to yield meaningful insights, yet manageable enough to facilitate logistics.
Employing both qualitative and quantitative measures enhances the depth and richness of the data collected, providing a more comprehensive view of the intervention's potential. Furthermore, involving stakeholders—like potential users and compliance representatives—can generate invaluable feedback that influences both device design and research protocols. Optimal methods require comprehensive documentation during the preliminary research process, ensuring compliance with regulatory standards.
By applying these methodologies, researchers can improve the efficiency and effectiveness of their preliminary trials, illustrating how pilot studies can accelerate medical device approval and ultimately speed up the approval process for innovative medical devices. As illustrated in the case analysis titled "How Pilot Studies Can Accelerate Medical Device Approval," which highlights bioaccess®'s role in optimizing research design, these pilot studies help identify potential issues in design and implementation, thereby optimizing resource allocation and reducing the risk of failed trials. Additionally, as Karen Becker Witkin, a Principal at the Weinberg Group, Inc., highlights in her forthcoming publication on research, the knowledge acquired from preliminary investigations is crucial in managing the intricacies of development programs, especially for Class III products, which are becoming more significant in today's medical environment.
Key Regulatory Considerations for Pilot Studies
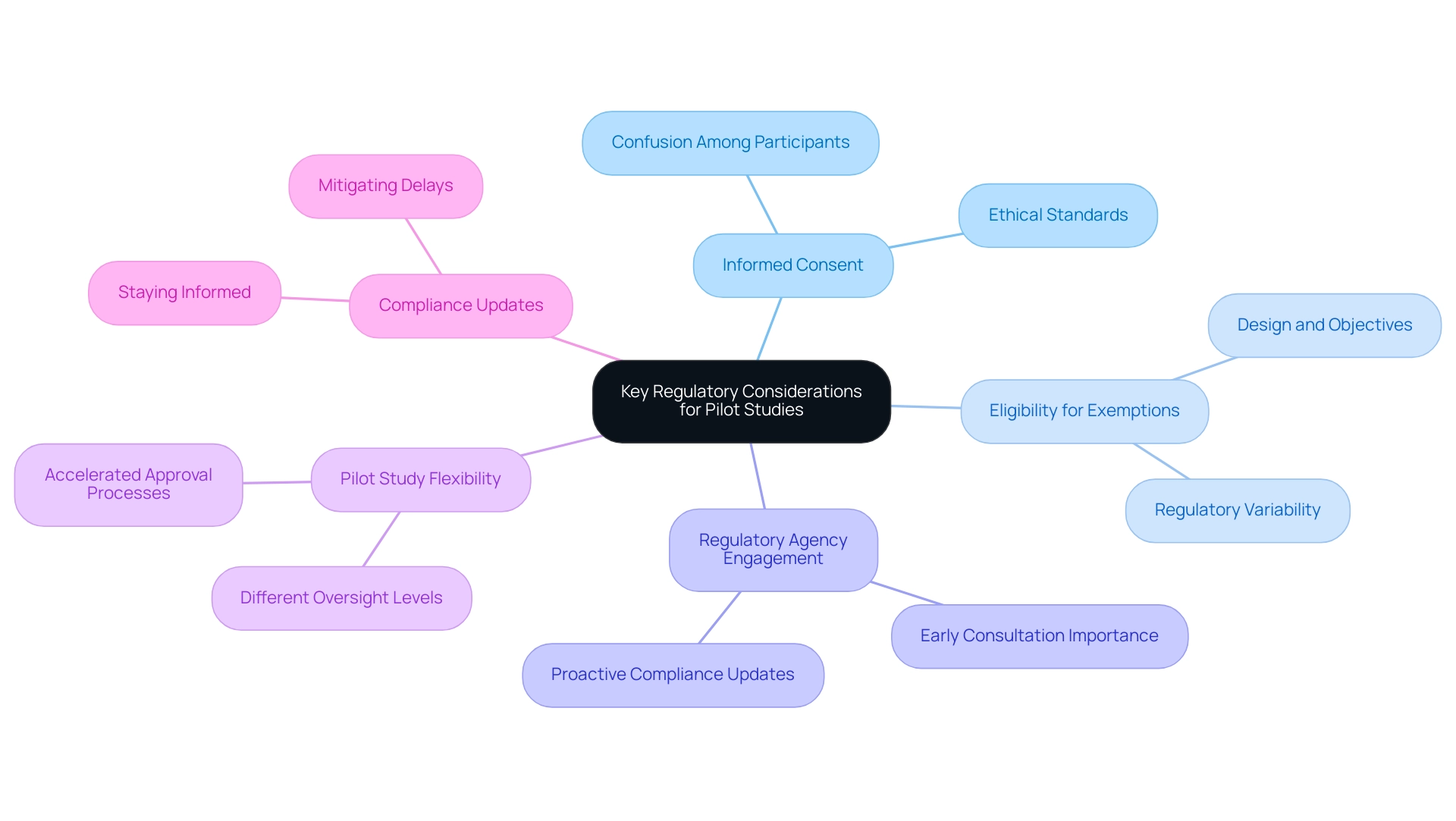
Following the guidelines set by entities like the FDA and EMA is crucial in the implementation of preliminary experiments. Researchers must meticulously design their pilot projects to comply with specific regulatory requirements, including obtaining informed consent from participants—a critical aspect underscored by recent statistics indicating that nearly 30% of participants in clinical trials report confusion regarding informed consent processes. Upholding ethical standards throughout the research demonstrates a commitment to participant welfare and data integrity.
Determining eligibility for exemption from certain regulations is also vital, as this may vary depending on the design and objectives of the research. Understanding the nuances that distinguish how pilot studies can accelerate medical device approval from larger clinical trials is essential; pilot studies often operate under different oversight levels, providing flexibility in their execution. For example, the FDA's guidance on Multiregional Clinical Trials (MRCTs) seeks to improve patient recruitment and retention while maintaining the integrity of trial results across various populations, highlighting the significance of compliance considerations.
Staying informed about compliance updates and engaging proactively with oversight bodies can mitigate potential delays and streamline the approval process. As emphasized by Katherine Ruiz, a specialist in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, early consultation with regulatory agencies such as the FDA is essential for ensuring compliance and efficacy in the development of biotechnology drugs and biosimilars, highlighting how pilot studies can accelerate medical device approval. Bioaccess® brings over 20 years of Medtech expertise and a tailored approach to managing these projects, including Early-Feasibility Assessments (EFA) and First-In-Human Trials (FIH), ensuring successful navigation through the complexities of clinical research in Latin America.
Challenges and Limitations of Pilot Studies
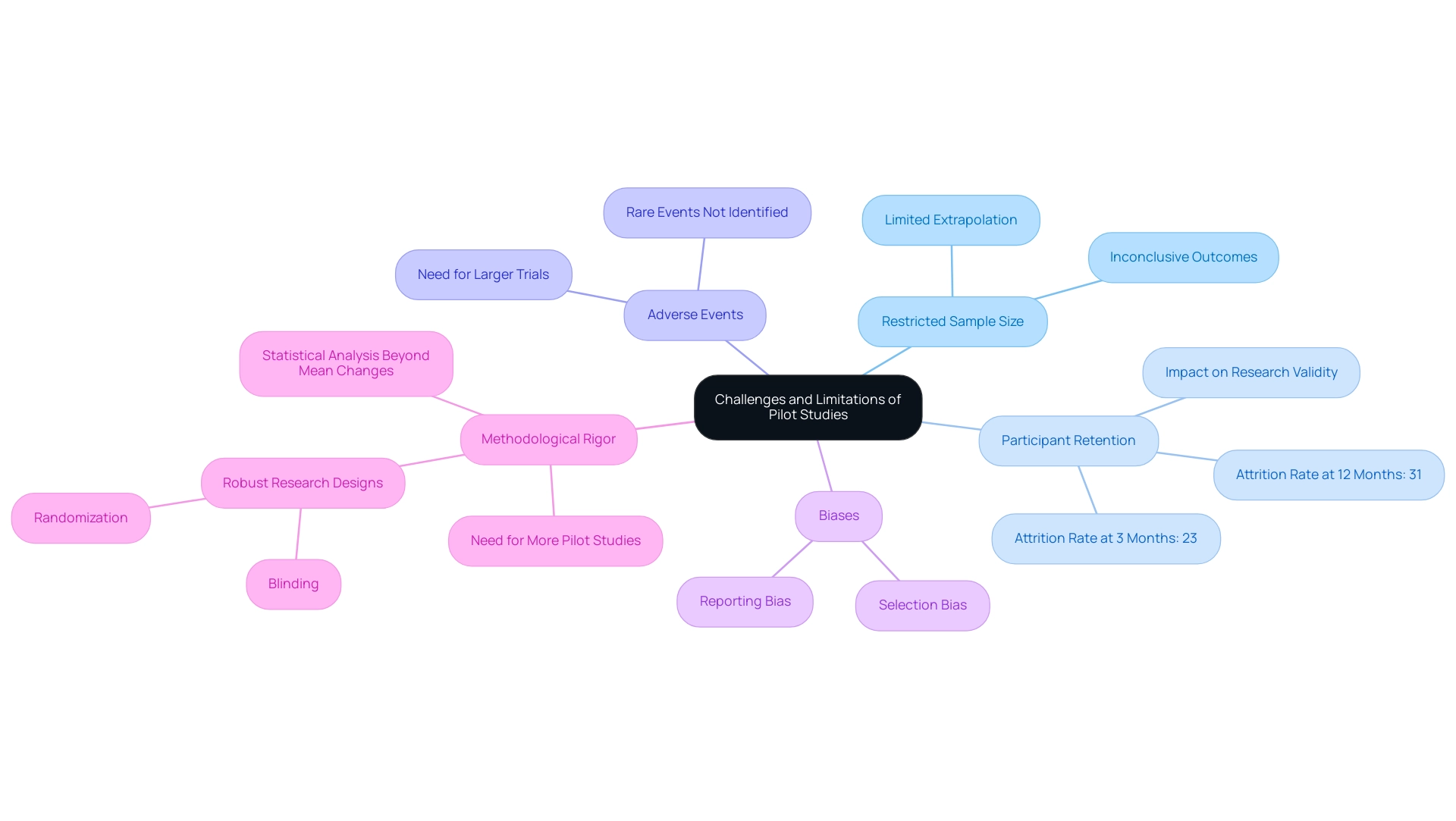
Pilot experiments are essential in the medical device approval process, illustrating how pilot studies can accelerate medical device approval, although they also come with several significant challenges. One of the foremost limitations is the restricted sample size, which frequently results in inconclusive outcomes that hinder the ability to extrapolate findings to larger populations. For instance, the attrition rate at three months was noted at 23%, a stark contrast to 31% at the twelve-month mark.
Such figures illustrate how participant retention can critically impact research validity. Furthermore, pilot investigations often fail to identify rare adverse events, which might only surface in larger clinical trials. As highlighted by Allen R. Kunselman, 'Pilot trials are not confirmatory experiments and therefore should not be hypothesis-driven (e.g., value-centric).'
This underscores the necessity for researchers to remain vigilant against biases that may cloud data interpretation, such as selection and reporting biases. To mitigate these challenges, implementing robust research designs is paramount, particularly in the context of how pilot studies can accelerate medical device approval; strategies like randomization and blinding should be prioritized wherever feasible. Additionally, researchers must be prepared for unexpected results, viewing these instances as opportunities to refine their hypotheses and study protocols in preparation for subsequent phases.
In the context of Latin America, the collaboration between bioaccess™ and the Caribbean Health Group exemplifies a strategic effort to enhance trial operations. Supported by Colombia's Minister of Health, this partnership aims to position Barranquilla as a leading destination for medical trials, leveraging local expertise and resources to enhance recruitment and retention rates. For example, the GlobalCare Clinical Trials collaboration with bioaccess™ has accomplished over a 50% decrease in recruitment time and 95% retention rates—showcasing the efficacy of well-managed trial phases.
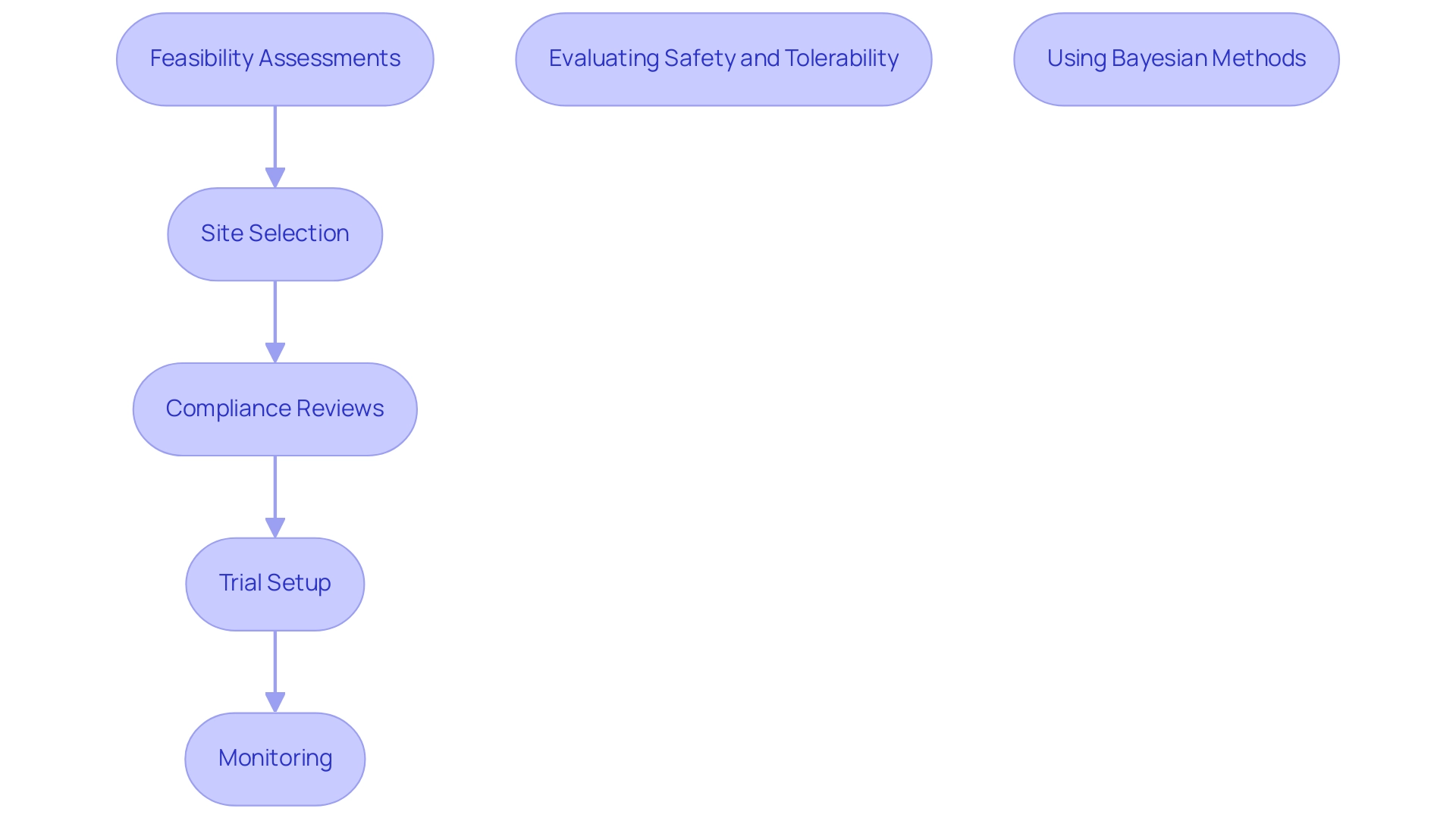
bioaccess™ specializes in managing initial trials and offers comprehensive clinical trial management services, including feasibility assessments, site selection, compliance reviews, trial setup, import permits, project management, and reporting. An evaluation of the methodological quality of preliminary investigations revealed that many fail to adequately address these issues, highlighting the need for enhanced methodological rigor and the publication of more initial research to substantiate efficacy potential—crucial for securing funding for subsequent larger trials, which underscores how pilot studies can accelerate medical device approval. Statistical analysis in initial experiments should go beyond mean changes to enhance data interpretability and provide preliminary treatment estimates.
Furthermore, logistic regression analyses have shown no statistically significant relationship between effect size and follow-up status of the research, further demonstrating the complexities and challenges encountered in preliminary investigations.
Best Practices for Conducting Successful Pilot Studies
To carry out successful preliminary investigations in Latin America, especially within the regulatory framework of Colombia, researchers must define clear objectives and hypotheses that correspond with both local and international regulatory expectations. The comprehensive process for advancing medical device trials includes crucial steps such as:
- Feasibility assessments
- Site selection
- Compliance reviews
- Trial setup
- Monitoring
These steps are essential for obtaining IRB/EC approval, INVIMA approval, and the necessary MinCIT import permits. Frequent misapplications of preliminary research include:
- Evaluating safety and tolerability
- Testing research hypotheses
- Estimating effect sizes for larger investigations
Engaging a multidisciplinary team significantly enhances research design by incorporating diverse perspectives, leading to more robust outcomes. Effective communication with stakeholders throughout the trial process is essential for collecting constructive feedback and ensuring alignment with regulatory requirements. Moreover, keeping detailed records of all research procedures and outcomes promotes transparency and reproducibility, which are vital in medical investigations.
An example of best practices can be seen in how pilot studies can accelerate medical device approval by using Bayesian methods, allowing researchers to integrate prior beliefs with observed data, thus informing decisions about larger trials. This approach not only aids in establishing Go/No-Go criteria for clinical development but also demonstrates how pilot studies can accelerate medical device approval while balancing the risks of false positives and negatives. Lastly, conducting thorough statistical analyses, preferably using parametric tests unless strong evidence of normality violation is present, will yield critical insights that guide the next steps in the regulatory process.
As Maria Fernandez stated, she 'contributed to the drafting and final approval of the manuscript,' highlighting the collaborative effort required in these studies, supported by the expertise and tailored services offered by bioaccess®. To learn more about how we can assist you, BOOK A MEETING with our team.
Conclusion
Pilot studies are an essential stepping stone in the medical device development process, playing a crucial role in ensuring the feasibility and safety of new technologies before they enter larger clinical trials. By allowing researchers to assess device practicality in real-world settings, pilot studies help identify potential challenges early, thereby streamlining the path to regulatory approval. The methodologies employed in these studies, combined with the insights gained, significantly enhance the likelihood of successful outcomes, ultimately benefiting both developers and patients.
Despite the advantages, pilot studies are not without their challenges. Limitations such as small sample sizes and the potential for inconclusive results can hinder the generalizability of findings. However, by adhering to best practices—such as robust study designs, thorough documentation, and stakeholder engagement—researchers can mitigate these obstacles and optimize the pilot study process. The collaboration between industry experts and regulatory bodies further strengthens the framework within which these studies operate, ensuring compliance and efficacy.
In summary, the importance of pilot studies in medical device research cannot be overstated. They serve not only as a foundation for the development of innovative technologies but also as a critical component in safeguarding patient safety and enhancing the overall success of clinical trials. As the landscape of medical device development continues to evolve, the insights gained from pilot studies will remain pivotal in navigating the complexities of regulatory approval and advancing healthcare solutions.
Frequently Asked Questions
What are pilot investigations in the context of medical device approval?
Pilot investigations, or feasibility assessments, are initial examinations that assess the viability, duration, expense, and potential negative occurrences linked with larger research trials for medical devices.
How do pilot studies accelerate medical device approval?
Pilot studies allow developers to assess the practicality of their devices in real-world settings, identify potential challenges early, and streamline the path to market by ensuring devices meet required standards.
What role does bioaccess™ play in pilot studies?
Bioaccess™ provides essential services such as selecting a principal investigator and submitting compliance dossiers, which support the approval process for medical devices.
What are some benefits of conducting pilot studies?
Pilot studies enhance device designs, gather preliminary safety and efficacy data, and help manufacturers save time, money, and resources while improving the success rates of clinical trials.
What types of studies are included in pilot investigations?
Pilot investigations can include early-feasibility studies, first-in-human studies, pilot studies, pivotal studies, and post-market clinical follow-up studies.
What methodologies are recommended for conducting effective pilot studies?
Recommended methodologies include choosing an appropriate sample size, employing both qualitative and quantitative measures, involving stakeholders for feedback, and ensuring comprehensive documentation for regulatory compliance.
Why is stakeholder involvement important in pilot studies?
Involving stakeholders, such as potential users and compliance representatives, generates valuable feedback that can influence device design and research protocols.
What challenges exist regarding the reliability of pilot studies?
There is ongoing discussion about whether pilot studies can reliably assess the safety or tolerability of a treatment, as comprehensive accounts on their effectiveness are limited in research literature.
How do preliminary investigations contribute to regulatory decisions?
Insights from preliminary investigations inform device development and play a crucial role in shaping regulatory decisions, enhancing the overall efficacy of clinical trials.
What is the significance of pilot studies in the context of Class III products?
Knowledge acquired from pilot studies is crucial for managing the complexities of development programs for Class III products, which are increasingly significant in the medical environment today.

