Introduction
The development of medical devices hinges on a critical aspect known as biocompatibility testing, which assesses how these devices interact with biological systems. This evaluation is vital for ensuring that medical devices do not provoke adverse reactions upon contact with human tissues, thereby safeguarding patient safety and enhancing treatment efficacy. As regulatory bodies like the FDA enforce stringent guidelines for biocompatibility assessments, manufacturers are compelled to adopt rigorous testing protocols.
With the Indian medical device testing market projected to experience substantial growth, the importance of biocompatibility testing becomes increasingly pronounced, especially as advancements in technology and regulatory frameworks evolve. This article delves into the foundational elements of biocompatibility testing, exploring key methodologies, regulatory standards, and emerging trends that shape the landscape of medical device development.
Introduction to Biocompatibility Testing for Medical Devices
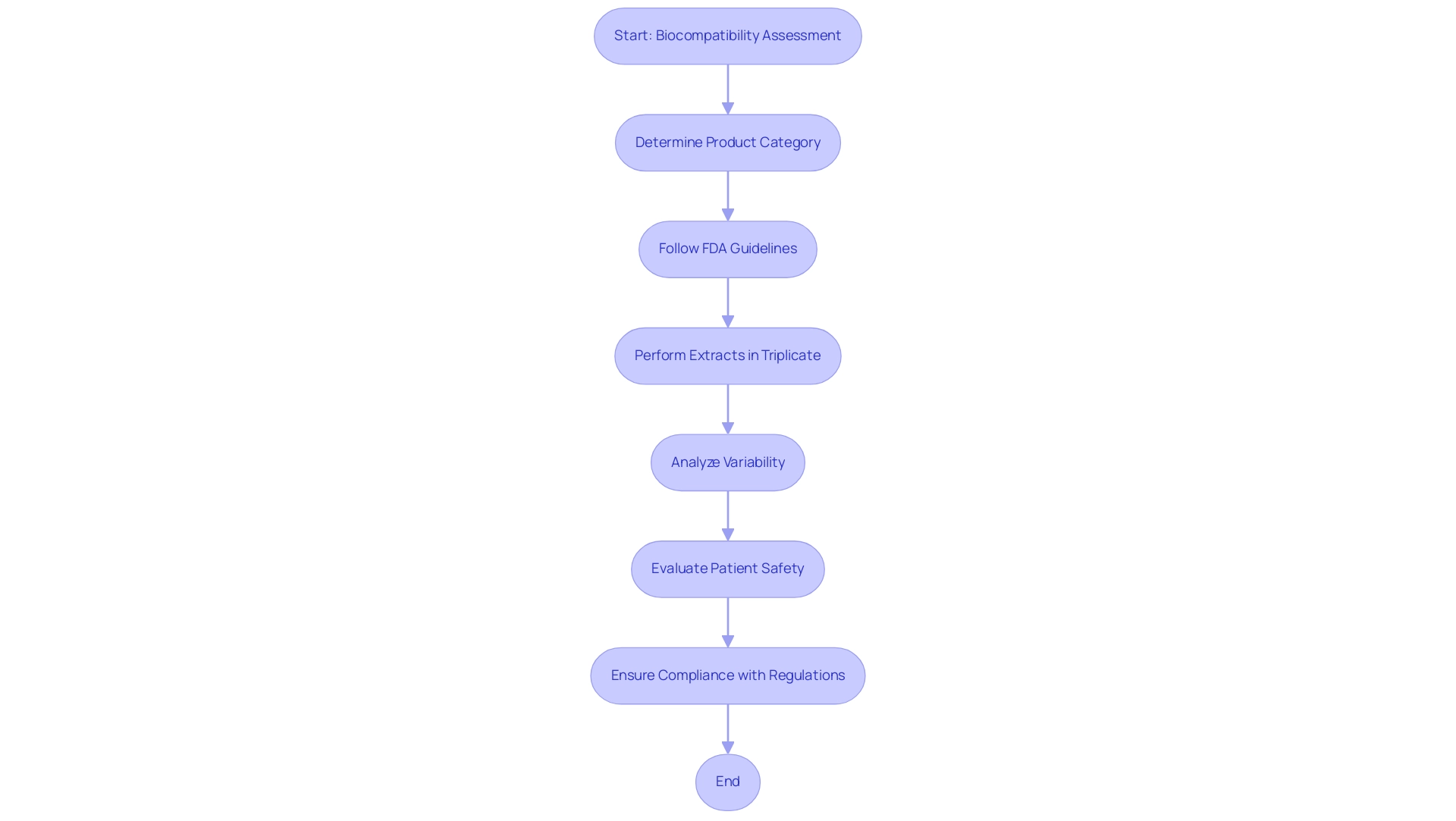
The assessment of medical device biocompatibility plays a crucial role in the advancement of healthcare instruments, as it examines the interaction between a product and biological systems. This assessment determines whether a gadget, upon contact with human tissues, may provoke any negative responses that could jeopardize patient safety. The essential nature of compatibility evaluation is highlighted by its direct impact on patient results and the overall effectiveness of medical procedures.
Regulatory bodies, particularly the Food and Drug Administration (FDA), require thorough assessments of compatibility to ensure that products comply with strict safety standards before their launch into the market and clinical application. According to the FDA,
For reliable results, it is recommended to perform extractions in triplicate for each solvent used, unless otherwise justified.
This guideline not only strengthens the requirement for thorough examination protocols but also aids in statistical analysis and assists in assessing variability, especially for products that may exhibit irregular manufacturing profiles.
With the Indian healthcare equipment evaluation and certification market anticipated to expand at a CAGR of 5.2%, reaching $2,388.4 million by 2029, the focus on compatibility with living organisms is becoming increasingly crucial. This growth emphasizes the importance of assessing medical device biocompatibility in ensuring patient safety and effective medical interventions. Certain product categories, including reprocessed single-use items, hydrogels, and adhesives, will necessitate comprehensive testing, reflecting ongoing advancements and regulatory updates in compatibility evaluations.
For instance, reprocessed single-use instruments pose unique challenges in ensuring medical device biocompatibility is maintained after they have been used and sterilized again. Additionally, the case study on the number of extraction replicates illustrates the practical application of the FDA's guidelines, emphasizing that triplicate extractions ensure a representative chemical profile and provide assurance of consistency, especially for products with potential variability in manufacturing.
Key Testing Methods and Standards: ISO 10993 Overview
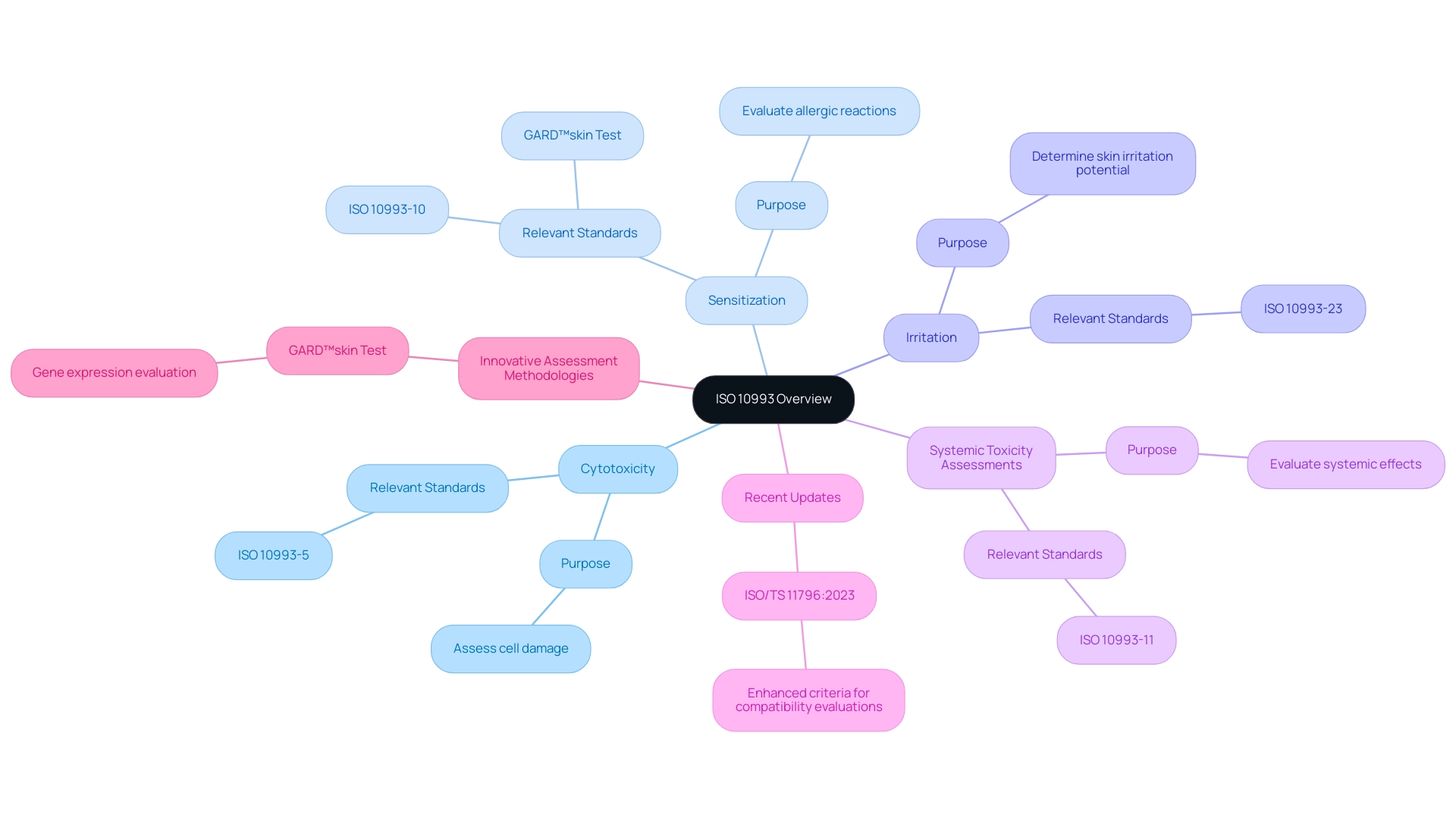
ISO 10993 represents a comprehensive series of international standards that delineate the guidelines for the medical device biocompatibility and biological evaluation of healthcare instruments. This framework includes a range of evaluation methods, such as:
- Cytotoxicity
- Sensitization
- Irritation
- Systemic toxicity assessments
Each method is carefully crafted to examine specific interactions between healthcare instruments and biological tissues. Compliance with ISO 10993 is not merely a regulatory obligation; it serves as a vital benchmark for manufacturers to evaluate medical device biocompatibility, ensuring that their products are both safe and effective for human use.
Recent discussions among experts highlight a critical gap in the adoption of innovative assessment methodologies. Kerecman Mayers et al. Despite significant advancements in the chemical sector and the inclusion of various techniques into OECD Test Guidelines informed by knowledge of key events leading to sensitisation, the medical device industry has not yet integrated these in vitro and in chemico methods into the ISO 10993 standards, which is crucial for ensuring medical device biocompatibility, as it still depends on animal trials for decision-making.
Significantly, the most recent updates feature ISO/TS 11796:2023, Edition 1, which further enhances the criteria for compatibility evaluations. Furthermore, the GARD™skin assessment (Project 4.106) illustrates modern progress in compatibility analysis by detecting skin sensitizers via gene expression evaluation in human dendritic cell-like cells. As the terrain of medical device biocompatibility evaluation advances, comprehending these standards becomes crucial for specialists involved in the testing and approval procedures of health-related products.
The Biological Evaluation Process: Steps and Considerations
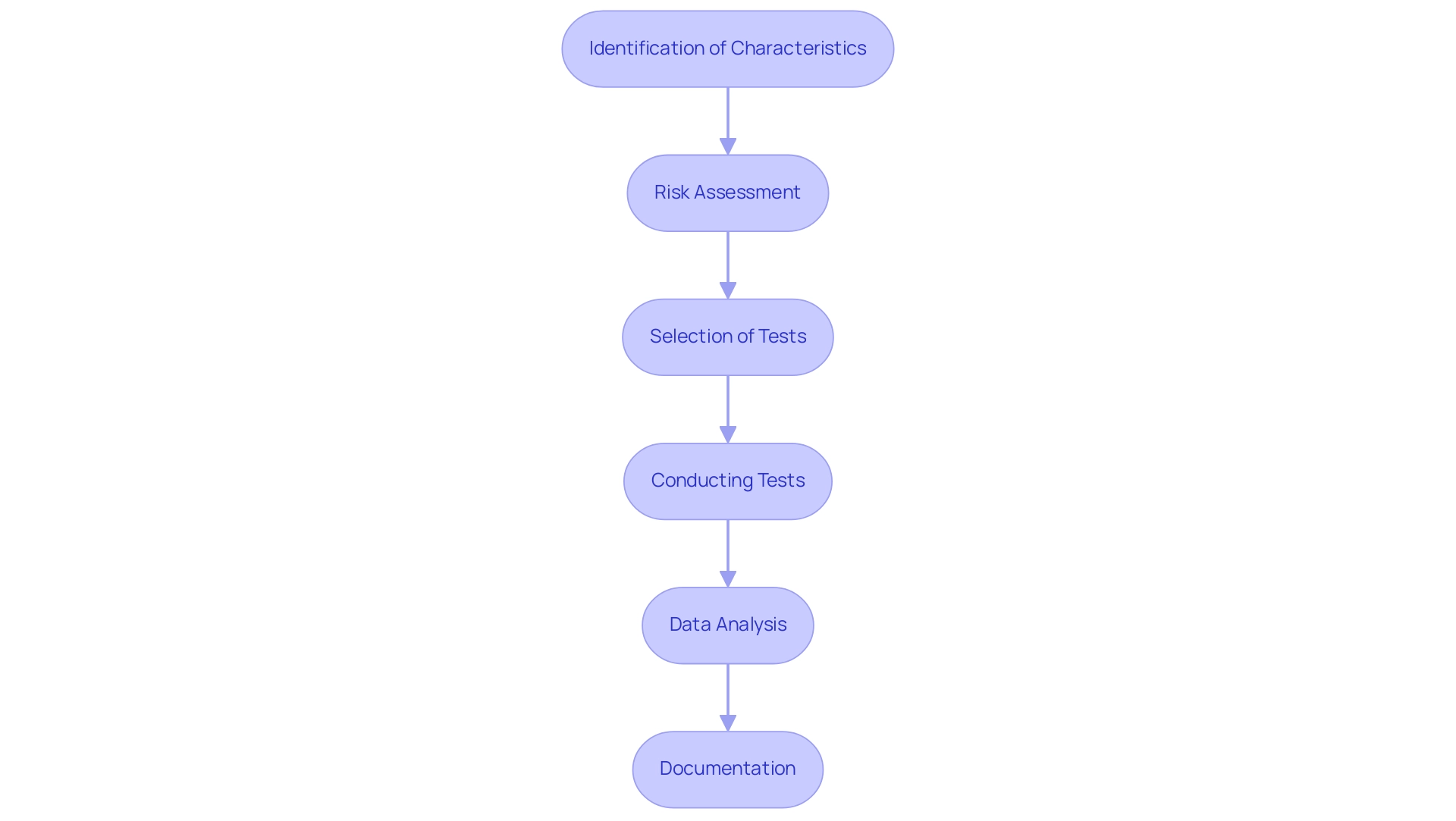
The biological evaluation process for medical devices is a systematic approach that encompasses several essential steps:
- Identification of Characteristics: Begin by understanding the materials utilized in the equipment and its intended application, as these factors significantly influence the evaluation process.
- Risk Assessment: A comprehensive risk evaluation is crucial for identifying potential hazards associated with the apparatus. As noted in current practices, small statistic values may suggest consistency with the null hypothesis, while larger values indicate less consistency, emphasizing the need for careful evaluation. An observed test statistic that falls in one tail at exactly the threshold has a value of 0.05, which highlights the significance of statistical thresholds in risk assessment.
- Selection of Tests: Based on the findings from the risk assessment, choose the suitable ISO 10993 tests designed to effectively assess the suitability of the product.
- Conducting Tests: Execute the selected tests in strict accordance with ISO 10993 protocols, gathering pertinent data on the biological responses elicited by the apparatus.
- Data Analysis: Examine the results thoroughly to determine the suitability of the apparatus. For instance, Kaplan-Meier outcomes plots comparing the times of contractile ring constriction in different cell populations demonstrated statistically significant differences, as determined by a log-rank test with p-values less than 0.0001, as noted by Goss et al. (2014), underlining the importance of robust statistical analysis.
- Documentation: Finally, prepare comprehensive documentation that substantiates the biocompatibility claims, ensuring that all findings are well-supported for regulatory submissions. This thorough evaluation process not only identifies and mitigates potential risks but also plays a pivotal role in ensuring medical device biocompatibility for the development of safe and effective health products. Furthermore, as highlighted in the case study titled "Designing Experiments with Statistical Analysis in Mind," integrating statistical analysis into the experimental design process is essential for yielding valid and interpretable results.
Impact of Biocompatibility Testing on Device Design and Compliance
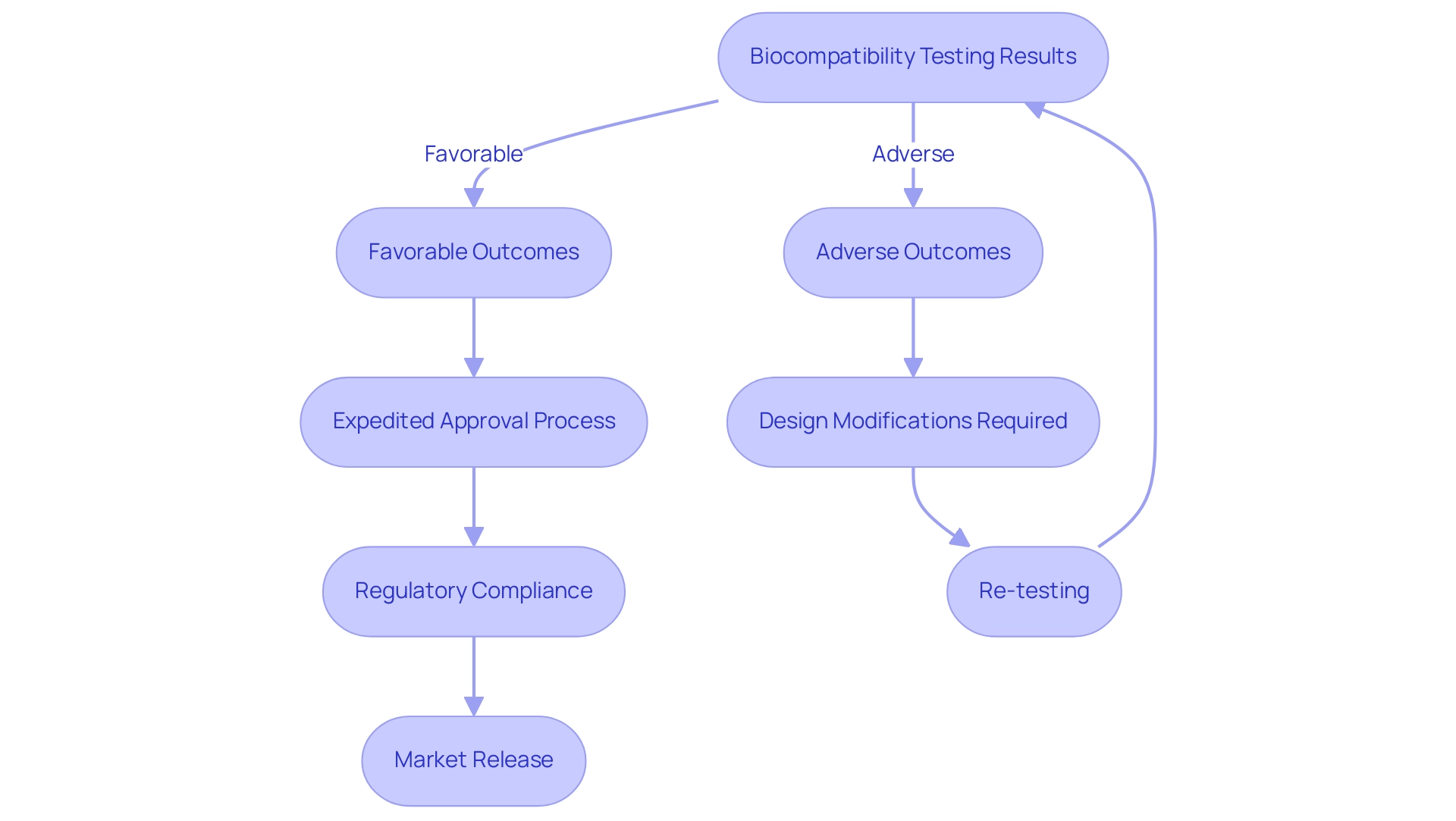
Biocompatibility testing produces considerable implications for the design and regulatory adherence of health-related products in Colombia, especially under the supervision of INVIMA (Colombia National Food and Drug Surveillance Institute). The Directorate for Medical Devices and other Technologies within INVIMA plays a crucial role in establishing and enforcing regulations related to medical devices, ensuring that they meet safety and efficacy standards. Favorable test outcomes can expedite the approval process, while adverse results often necessitate crucial design modifications to address identified risks.
For instance, Rathore et al. discussed dental amalgam toxicity, emphasizing the significance of evaluating safety to prevent potential health hazards. Given INVIMA's classification as a Level 4 health authority by PAHO/WHO, it is essential for manufacturers to incorporate safety considerations early in the design phase, as this proactive approach can prevent costly alterations later in the development cycle.
Adhering to established compatibility standards not only aids in regulatory approval from INVIMA but also boosts stakeholder confidence in the safety and effectiveness of the device. Katherine Ruiz, a specialist in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, stresses that compatibility evaluations go beyond being just a regulatory formality; it is a crucial component of the product development lifecycle, profoundly impacting design choices, evaluation procedures, and the overall schedule of projects. Moreover, entities such as ISO, FDA, and TÜV SÜD play a vital role in the regulatory process, ensuring adherence to safety regulations through established evaluation criteria.
By adhering to these standards and utilizing test results related to compatibility within risk management strategies, developers can not only enhance patient outcomes but also promote innovative healthcare technologies.
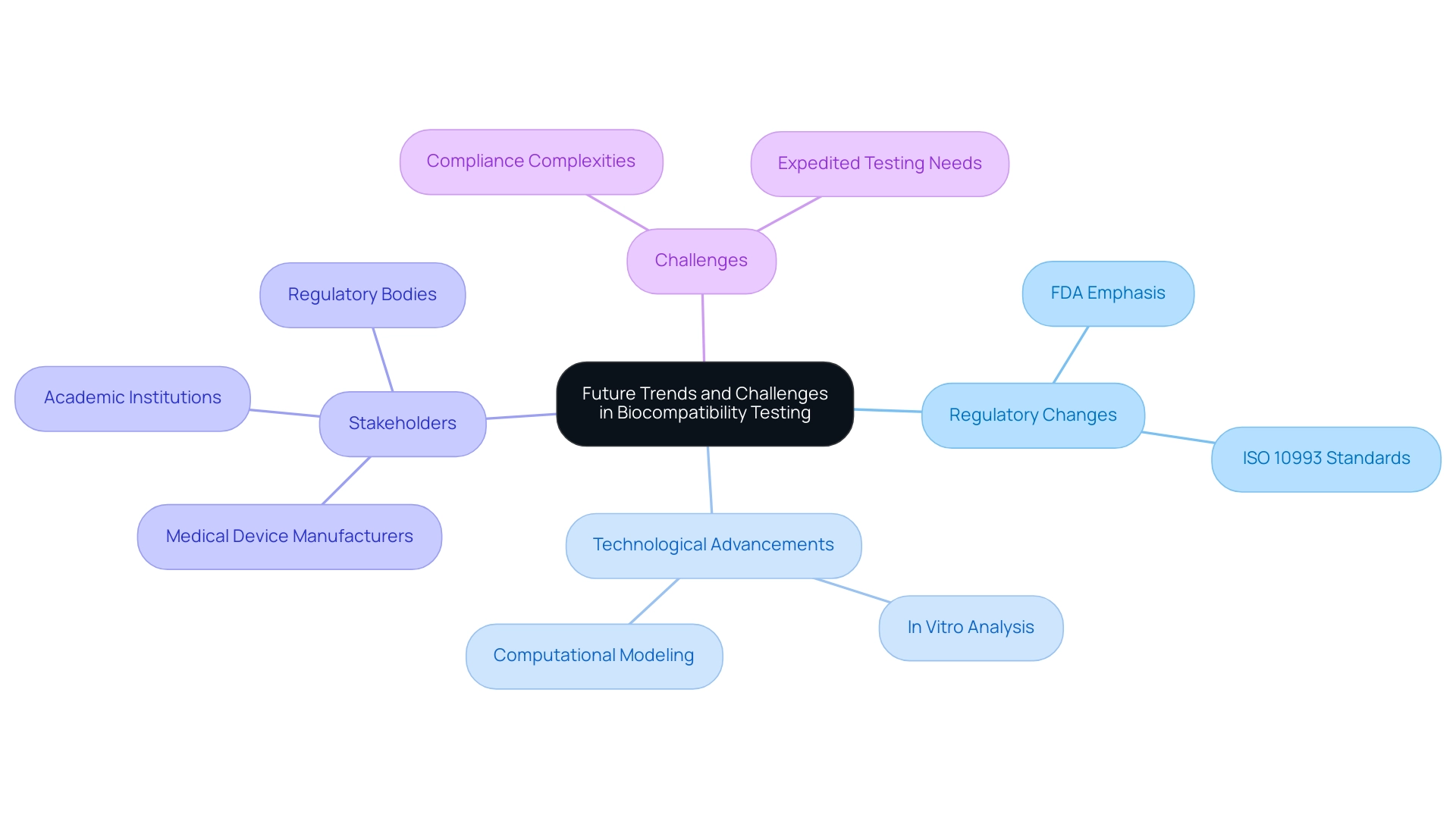
Future Trends and Challenges in Biocompatibility Testing
The area of medical device biocompatibility evaluation is undergoing significant transformation, driven by evolving regulatory requirements and technological advancements. Led by experts like Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, and Katherine Ruiz, a specialist in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, the industry is adapting to new standards. Regulatory bodies, including the FDA, are placing a stronger emphasis on innovative evaluation methods aimed at reducing reliance on animal experimentation while enhancing efficiency.
As we approach 2024, the integration of advanced technologies such as in vitro analysis and computational modeling is increasingly prevalent, offering promising alternatives for traditional evaluation methods. Recently, a contribution was made regarding the enforcement of lab safety protocols, highlighting the ongoing efforts to ensure compliance and safety within examination environments. However, this shift is not without its challenges.
Professionals must navigate the complexities of new regulations, ensure compliance with the latest ISO 10993 standards, and meet the rising demand for expedited testing solutions, all while ensuring medical device biocompatibility and safety. These trends and challenges underscore the necessity for stakeholders—ranging from medical device manufacturers to academic institutions—to stay informed and adapt their strategies accordingly. The geographical analysis of the market indicates that regions such as North America, Europe, Asia-Pacific, the Middle East and Africa, and South America are critical players in the industry landscape.
As noted by Nirav Gokani, a guest post author, 'The prospects described in the report assist the stakeholders and report buyers in properly planning their investments and obtaining the most return on investment.' This insight emphasizes the critical need for proactive engagement with these emerging trends to optimize assessments of medical device biocompatibility and regulatory submissions in the forthcoming years. Furthermore, the case study titled 'Enforcing Lab Safety Protocols' illustrates the real-world challenges faced by professionals in ensuring compliance with safety measures, which is essential for maintaining the integrity of medical device biocompatibility testing.
Conclusion
The significance of biocompatibility testing in the development of medical devices cannot be overstated. As outlined, this testing is essential for evaluating the interactions between medical devices and biological systems, ensuring that patient safety is prioritized. With stringent regulatory mandates, particularly from bodies like the FDA, manufacturers are required to adhere to comprehensive testing protocols, which not only safeguard patient health but also enhance the efficacy of medical interventions.
The article detailed key methodologies and standards, notably the ISO 10993 framework, which serves as a benchmark for ensuring the safety and effectiveness of medical devices. It emphasized the systematic biological evaluation process, including:
- Risk assessment
- Test selection
- Thorough documentation
All of which are critical in substantiating biocompatibility claims. Furthermore, the implications of biocompatibility testing on device design highlight its integral role in regulatory compliance and stakeholder confidence.
Looking ahead, the evolving landscape of biocompatibility testing is marked by advancements in technology and regulatory frameworks. The shift towards innovative testing methods, such as:
- In vitro approaches
- Computational modeling
presents both opportunities and challenges for industry stakeholders. As the medical device market continues to grow, particularly in regions like India and Colombia, the importance of adapting to these trends while maintaining rigorous safety standards will be paramount.
Ultimately, the future of medical device development hinges on a steadfast commitment to biocompatibility testing. By prioritizing these evaluations, manufacturers can not only ensure compliance with regulatory standards but also contribute to the advancement of safer and more effective healthcare solutions.
Frequently Asked Questions
What is the importance of medical device biocompatibility assessment?
Medical device biocompatibility assessment is crucial as it examines the interaction between a device and biological systems, determining whether it may provoke negative responses upon contact with human tissues, thus ensuring patient safety and the effectiveness of medical procedures.
What role do regulatory bodies like the FDA play in biocompatibility assessments?
Regulatory bodies, particularly the FDA, require thorough biocompatibility assessments to ensure that medical devices comply with strict safety standards before they are launched into the market and used clinically.
What is the FDA's recommendation regarding extraction tests for biocompatibility?
The FDA recommends performing extractions in triplicate for each solvent used to ensure reliable results, which aids in statistical analysis and assesses variability, especially for products with irregular manufacturing profiles.
How is the Indian healthcare equipment evaluation market expected to grow, and what does this mean for biocompatibility assessments?
The Indian healthcare equipment evaluation market is anticipated to expand at a CAGR of 5.2%, reaching $2,388.4 million by 2029, emphasizing the increasing importance of assessing medical device biocompatibility for ensuring patient safety and effective medical interventions.
What specific product categories require comprehensive biocompatibility testing?
Product categories such as reprocessed single-use items, hydrogels, and adhesives necessitate comprehensive testing due to ongoing advancements and regulatory updates in compatibility evaluations.
What challenges do reprocessed single-use instruments present in terms of biocompatibility?
Reprocessed single-use instruments pose unique challenges in maintaining medical device biocompatibility after they have been used and sterilized again.
What does ISO 10993 represent in the context of medical device biocompatibility?
ISO 10993 represents a comprehensive series of international standards that provide guidelines for the biocompatibility and biological evaluation of healthcare instruments, including various evaluation methods such as cytotoxicity, sensitization, irritation, and systemic toxicity assessments.
Why is compliance with ISO 10993 important for manufacturers?
Compliance with ISO 10993 is vital for manufacturers as it serves as a benchmark to evaluate medical device biocompatibility, ensuring that their products are safe and effective for human use.
What recent updates have been made to the ISO standards regarding biocompatibility?
Recent updates include ISO/TS 11796:2023, which enhances the criteria for compatibility evaluations and introduces modern assessment methods like the GARD™skin assessment for detecting skin sensitizers.
What gap exists in the adoption of innovative assessment methodologies in the medical device industry?
There is a critical gap in the adoption of innovative assessment methodologies, as the medical device industry has not yet integrated in vitro and in chemico methods into ISO 10993 standards, still relying heavily on animal trials for decision-making.




