Introduction
The Medical Device Regulation (MDR) represents a transformative shift in the regulatory framework governing medical devices within the European Union, aimed at significantly enhancing patient safety and device efficacy. With its rigorous requirements for clinical evaluation and post-market surveillance, the MDR seeks to foster public trust in medical technologies and promote innovation in the healthcare sector.
This article delves into the essential components of the MDR, including its purpose, key compliance steps, the notable changes from previous regulations, and the challenges manufacturers face in adapting to these new standards. By examining these aspects, the discussion highlights the critical role of education and training in navigating the complexities of compliance, ultimately underscoring the importance of robust regulatory practices for the advancement of medical device safety and effectiveness.
Overview of the Medical Device Regulation (MDR): Purpose and Significance
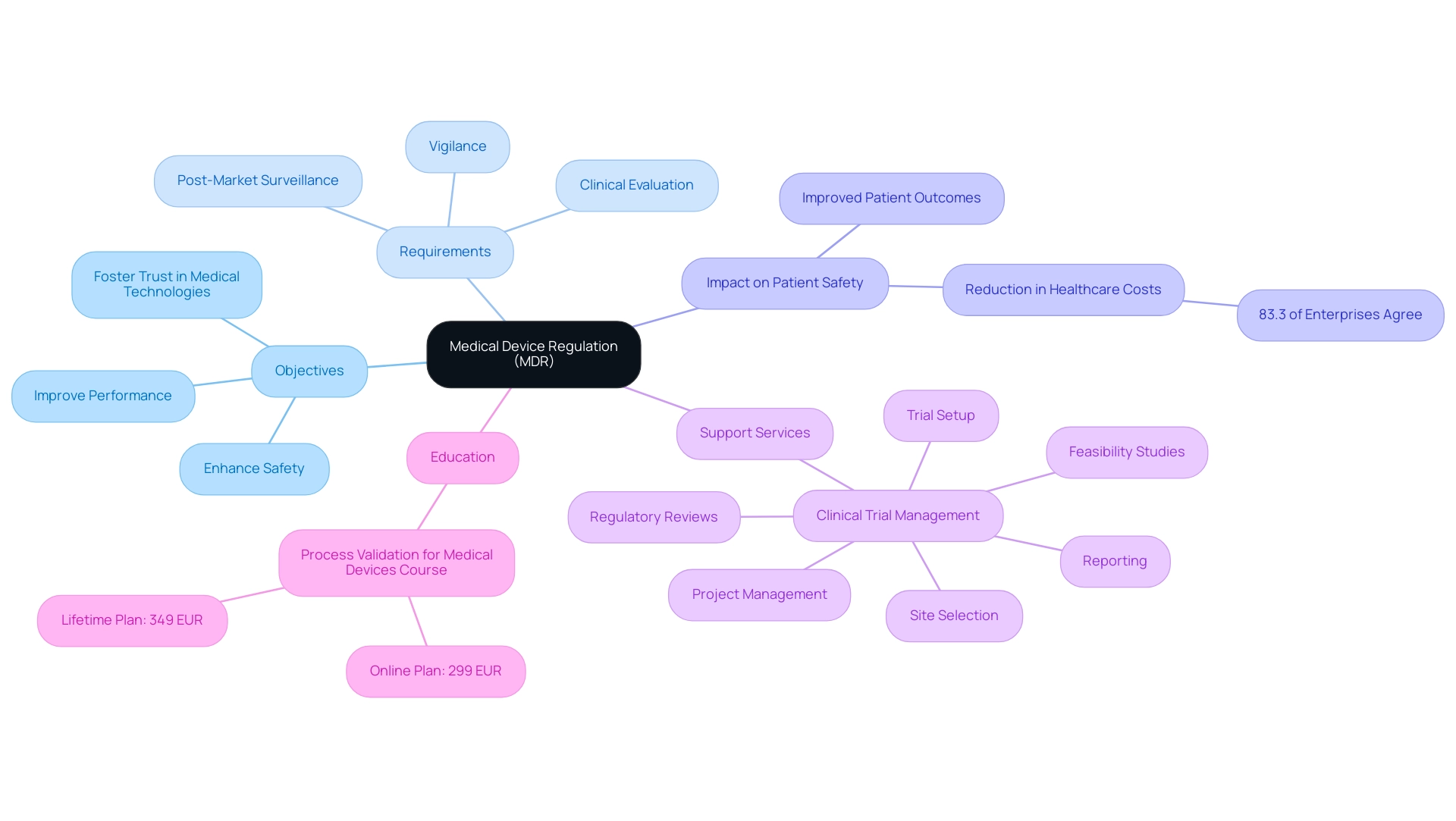
The medical device regulation MDR was introduced to significantly enhance the safety and performance of medical instruments within the European Union. Its primary objective is to enforce rigorous scrutiny of products prior to market entry, thereby safeguarding patient health. The medical device regulation MDR establishes comprehensive requirements that include:
- Clinical evaluation
- Post-market surveillance
- Vigilance
These are critical for ensuring that medical products meet high safety and efficacy standards.
As pointed out by medical equipment specialist Pontus Gedda, who has extensive experience with the medical device regulation MDR's implementation, this regulation is vital for fostering trust in medical technologies. By reinforcing public confidence, the MDR ultimately contributes to improved patient outcomes and supports innovation in the medical sector. A recent survey, which included responses from 68 MedTech professionals primarily in quality and Regulatory Affairs, revealed that 83.3% of large enterprises acknowledge the role of health technology in reducing healthcare costs, further illustrating the positive ripple effects of medical device regulation MDR on both patient safety and overall healthcare efficiency.
Additionally, the study titled 'Scientific Literature Reviews as Clinical Evidence' identified scientific literature reviews as a commonly used source of reactive post-market surveillance (PMS) data for clinical evidence, indicating that over 70% of respondents utilize MEDDEV 2.7/1 Rev 4 for conducting these reviews. This highlights the impact of medical device regulation MDR on patient safety by ensuring that the clinical evidence is robust and reliable. Moreover, with the knowledge of firms such as bioaccess®, which provides extensive clinical trial management services in Latin America, including:
- Feasibility studies
- Site selection
- Regulatory reviews
- Trial setup
- Project management
- Reporting
The area is prepared for substantial progress in medical innovation.
These services not only aid in adhering to the medical device regulation MDR but also contribute to local economies through job creation and healthcare improvements. Ongoing education in the field is exemplified by the recent launch of a Process Validation for Medical Equipment course, emphasizing the importance of continual learning and adaptation to regulatory changes in enhancing medical equipment safety.
Navigating Compliance: Key Steps and Resources for MDR Adherence
To successfully navigate compliance with medical device regulation MDR, manufacturers should adhere to the following critical steps:
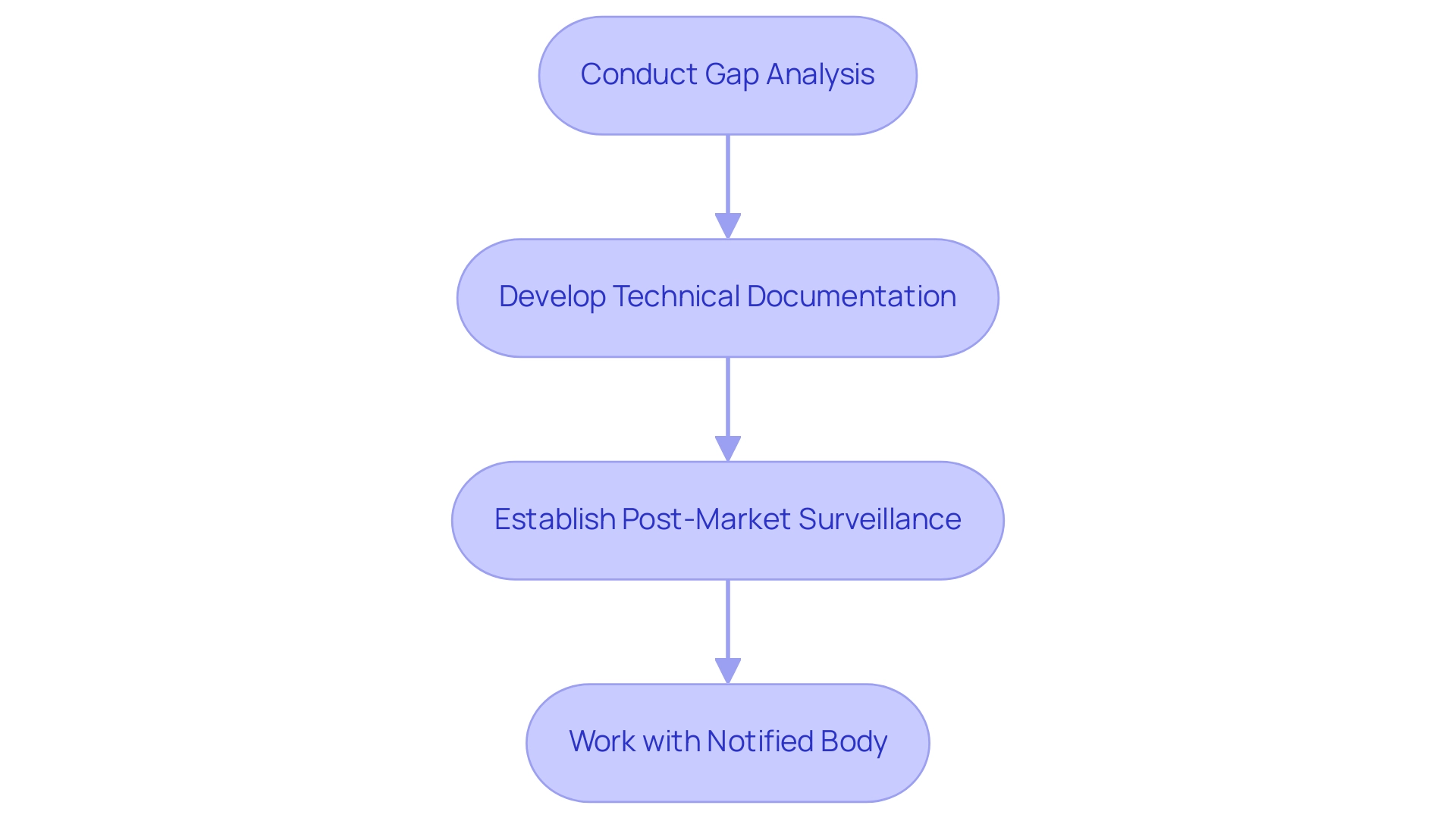
- Conduct a thorough gap analysis to identify discrepancies between existing practices and the medical device regulation MDR requirements.
- Develop and maintain a comprehensive Technical Documentation file that complies with medical device regulation MDR, including detailed risk assessments and robust clinical evaluations.
- Establish a post-market surveillance system designed to collect and analyze data regarding device performance after market introduction.
- Work with a Notified Body to assist the conformity assessment processes needed for medical device regulation MDR approval.
In the context of the Finnish health technology industry, where all large enterprises concur that health technology enhances healthcare effectiveness, it is essential for manufacturers to utilize resources such as the European Commission's guidance documents, checklists, and industry workshops to stay informed about the latest regulatory requirements and best practices.
Prominent specialists in the sector, such as Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, emphasize the significance of thorough clinical trial management services, which encompass feasibility studies, site selection, reviews, trial setup, import permits, project management, and reporting. Ana Criado states, "Our customized method for clinical trial management not only guarantees adherence but also improves the overall efficiency of the research process."
As Kaija Saranto from the University of Eastern Finland notes, "The integration of health technology is essential for enhancing operational effectiveness in the healthcare sector." This proactive approach not only aids in meeting compliance standards but also enhances overall operational effectiveness amidst the current challenges faced in the industry.
Key Changes in the MDR: Evolution from Previous Regulations
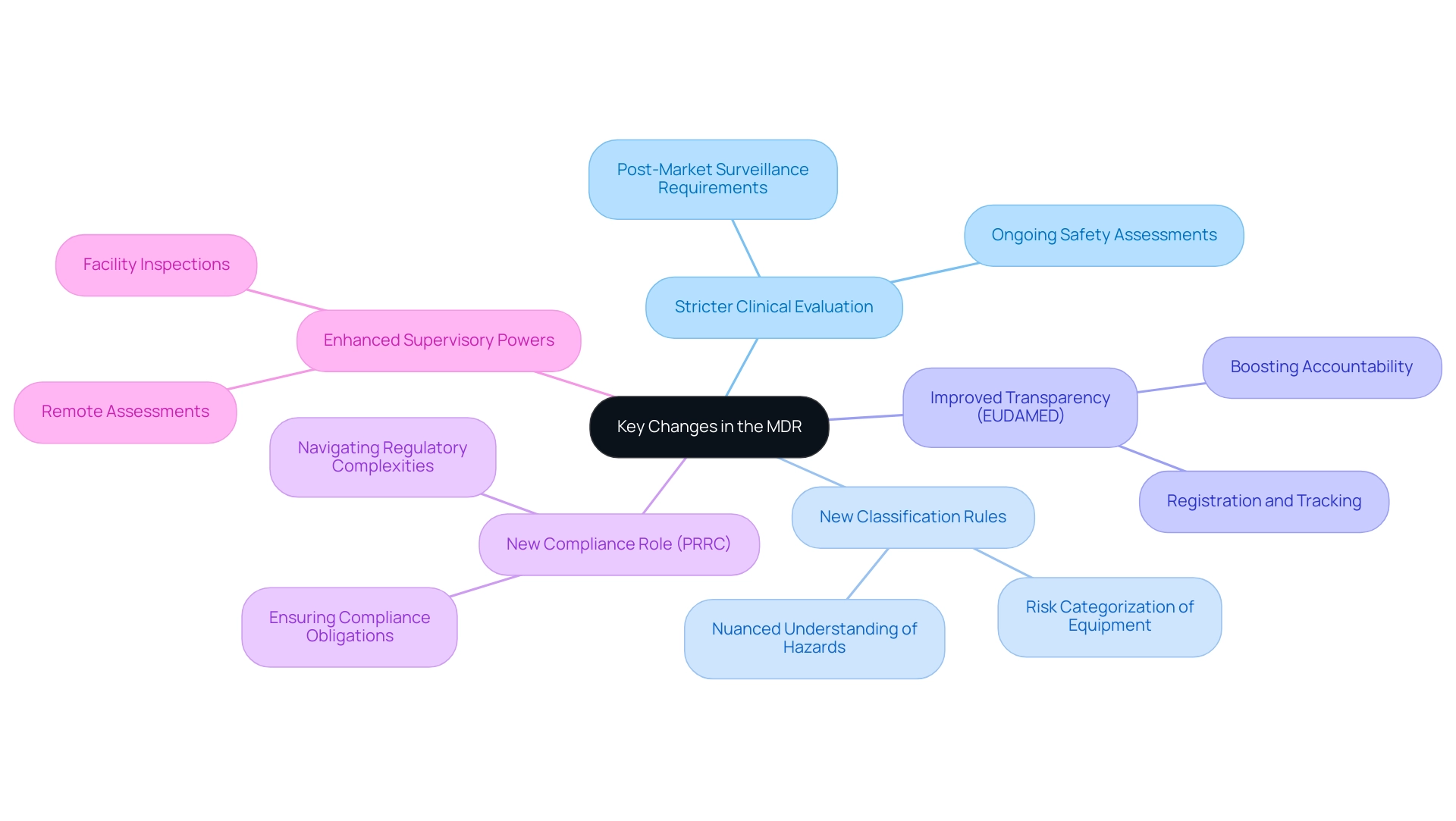
The medical device regulation MDR introduces several significant changes compared to the previous Medical Device Directive (MDD), marking a pivotal shift in the regulatory landscape. Among the most notable adjustments are:
- Stricter requirements for clinical evaluation and post-market surveillance, which are crucial for ongoing safety and efficacy assessments.
- New classification rules that directly influence the risk categorization of equipment, ensuring a more nuanced understanding of potential hazards.
- Improved transparency through the EUDAMED database, which aids in registration and tracking, thus boosting accountability within the industry. The introduction of this database has been met with positive expert feedback, noting its potential to bolster device transparency significantly.
- A new obligation for manufacturers to appoint a Person Responsible for Compliance (PRRC), ensuring that compliance obligations are met consistently and effectively. This role is critical in navigating the complexities of medical device regulation MDR.
- Improved supervisory power to carry out remote assessments and examine facilities involved in research on instruments, demonstrating a dedication to strict adherence.
As mentioned by Leiter and White, "medical instruments are increasingly being implanted in human bodies, constituting manufactured risks," emphasizing the critical need for these oversight changes. Furthermore, our service capabilities include:
- Feasibility and selection of research sites and principal investigators (PIs)
- Thorough reviews and feedback on study documents to ensure compliance with country requirements
- Comprehensive trial setup and management
This encompasses the thorough procedure of obtaining essential import permits and nationalizing investigational equipment, ensuring that all regulatory requirements are satisfied. Our approach also encompasses diligent project monitoring and reporting on study status and adverse events.
A recent case study on the costs associated with medical equipment development reveals that the total estimated expenses for developing these items can reach up to EUR 186,598, underscoring the financial implications that medical device regulation MDR entails.
These changes highlight a broader commitment to ensuring greater accountability and safety standards within the medical device sector, reflecting an evolving landscape where adherence is integral to market access and the potential for economic growth through local medtech studies.
Challenges in Adapting to the MDR: Insights from the Medtech Sector
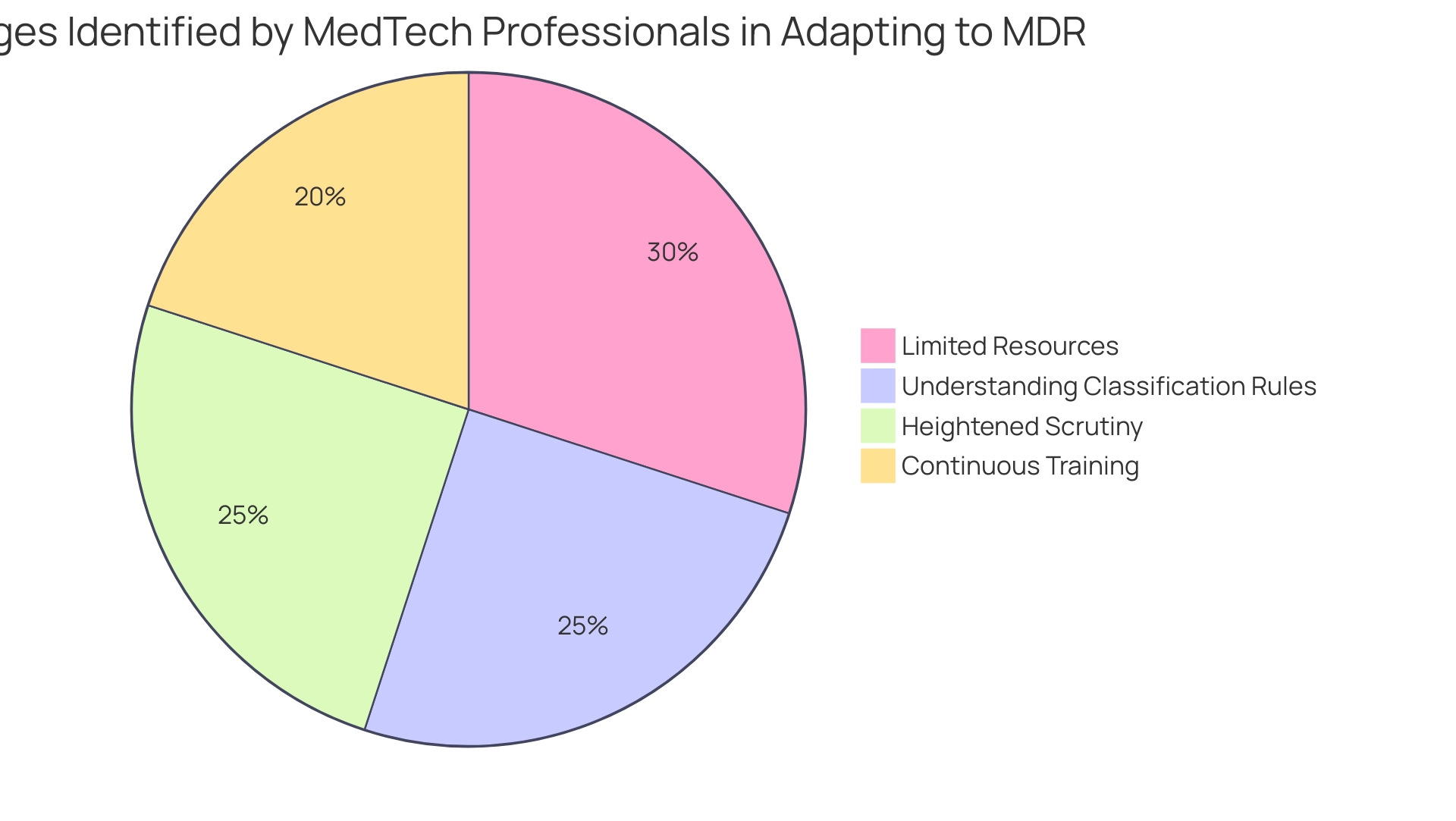
Manufacturers in the medtech sector encounter significant hurdles as they adapt to the medical device regulation MDR. Key challenges include:
- Limited resources and expertise for managing the substantial increase in documentation requirements.
- The complexities involved in understanding new classification rules and conducting thorough risk assessments.
- A continuous necessity for training and education to ensure that staff are well-versed in the latest compliance changes.
- Heightened scrutiny from Notified Bodies, resulting in extended review turnaround times, which can average between 12 to 15 months.
A recent quantitative study revealed that 85% of MedTech professionals identified these challenges when generating Clinical Evaluation Reports (CERs) under the medical device regulation MDR. Regina Preysing, a Partnerships Manager, emphasizes,
Having the right tools in place for QMS and documentation is a crucial success factor for the time to product marketing and thus for the business success of your company.
Moreover, the extensive services provided—including feasibility studies, site selection, trial set-up, start-up approvals, review processes, project management, and reporting of serious and non-serious adverse events—effectively tackle these obstacles.
Eric Hill's experience in managing projects like rebranding, CAPA remediation, and FDA responses emphasizes the multifaceted challenges that companies encounter in this evolving compliance landscape. Additionally, a study on the outsourcing of Clinical Evaluation Reports found that most respondents (76%) did not outsource CER generation, with a majority of those who did being large enterprises. This indicates a trend towards in-house evaluations as firms seek to better navigate medical device regulation MDR compliance.
To effectively tackle these obstacles, proactive planning, investment in comprehensive training, and collaboration with regulatory experts are essential strategies for success.
The Role of Education and Training in Ensuring MDR Compliance
Ongoing education and training are indispensable for all stakeholders involved in the medical device regulation MDR, particularly in light of the complexities introduced by it. Leaders like Ana Criado, our Director of Regulatory Affairs and a professor with extensive experience in biomedical engineering and health economics, exemplify the critical need for comprehensive training programs. Manufacturers must prioritize investments that encompass vital aspects of medical device regulation MDR compliance, risk management, and post-market surveillance.
Various educational formats, such as workshops, online courses, and certification programs, can offer valuable insights tailored to the evolving regulatory landscape. As highlighted by industry experts, 'for many moderate/high risk and innovative devices, PMCF is an PMS activity, and not just because it monitors the safety and performance of your device.' This highlights the significance of training in ensuring effective monitoring and adherence.
Cultivating a culture of continuous learning within organizations is vital, as it ensures teams remain abreast of regulatory updates and best practices. This proactive strategy not only improves adherence efforts but also cultivates a robust organizational structure capable of addressing the challenges posed by modern medical device regulation. Recent statistics indicate that organizations with structured training programs for medical device regulation MDR adherence see a significant increase in adherence rates, reinforcing the necessity of well-structured educational initiatives.
Furthermore, the emergence of Managed Detection and Response (MDR) as a comprehensive solution for compliance challenges highlights the critical need for organizations to adopt effective training and monitoring strategies. By investing in education and training, medical device manufacturers can better manage the complexities of medical device regulation (MDR) adherence, guided by experts like Ana Criado and Katherine Ruiz, who specializes in oversight for medical devices and in vitro diagnostics in Colombia. Katherine's insights into the regulatory landscape further enhance the training initiatives, ensuring a well-rounded approach to compliance.
Conclusion
The Medical Device Regulation (MDR) marks a significant evolution in the regulatory framework for medical devices within the European Union, emphasizing the paramount importance of patient safety and device efficacy. By instituting stringent requirements for clinical evaluation and post-market surveillance, the MDR not only enhances public trust in medical technologies but also fosters innovation in the healthcare sector. The integration of comprehensive compliance steps, such as thorough gap analyses and collaboration with Notified Bodies, is essential for manufacturers aiming to meet these new standards.
Transitioning from the previous Medical Device Directive (MDD), the MDR introduces critical changes, including new classification rules and enhanced transparency through the EUDAMED database. These modifications necessitate a proactive approach from manufacturers, who must navigate the complexities of compliance while addressing challenges such as increased documentation demands and the need for specialized training.
Ongoing education and training emerge as vital components in successfully adapting to the MDR landscape. By prioritizing investments in training programs and fostering a culture of continuous learning, organizations can significantly improve their compliance rates. The commitment to education not only equips teams with the necessary knowledge to navigate regulatory complexities but also underlines the importance of robust regulatory practices in advancing medical device safety and effectiveness. In summary, the MDR represents a transformative opportunity for the medical device sector to enhance patient outcomes while driving innovation through rigorous compliance and education.
Frequently Asked Questions
What is the purpose of the Medical Device Regulation (MDR) in the European Union?
The MDR aims to enhance the safety and performance of medical instruments by enforcing rigorous scrutiny of products before they enter the market, thereby safeguarding patient health.
What are the key requirements established by the MDR?
The MDR establishes comprehensive requirements that include clinical evaluation, post-market surveillance, and vigilance to ensure that medical products meet high safety and efficacy standards.
How does the MDR contribute to public trust in medical technologies?
By reinforcing public confidence in medical technologies, the MDR fosters trust, which ultimately contributes to improved patient outcomes and supports innovation in the medical sector.
What percentage of large enterprises acknowledge the role of health technology in reducing healthcare costs?
A recent survey revealed that 83.3% of large enterprises acknowledge the role of health technology in reducing healthcare costs.
What is the significance of scientific literature reviews in the context of the MDR?
Scientific literature reviews are a commonly used source of reactive post-market surveillance data for clinical evidence, with over 70% of respondents utilizing MEDDEV 2.7/1 Rev 4 for these reviews, ensuring robust and reliable clinical evidence.
What services does bioaccess® provide in relation to the MDR?
Bioaccess® offers extensive clinical trial management services, including feasibility studies, site selection, regulatory reviews, trial setup, project management, and reporting, which help adhere to the MDR and contribute to local economies.
What ongoing education initiatives are available for professionals in the medical device field?
Recent educational initiatives, such as the launch of a Process Validation for Medical Equipment course, emphasize the importance of continual learning and adaptation to regulatory changes to enhance medical equipment safety.
What are the critical steps manufacturers should follow to comply with the MDR?
Manufacturers should conduct a gap analysis, develop comprehensive Technical Documentation, establish a post-market surveillance system, and work with a Notified Body for conformity assessment processes.
How can manufacturers stay informed about the latest regulatory requirements and best practices?
Manufacturers can utilize resources such as the European Commission's guidance documents, checklists, and industry workshops to stay informed about the latest regulatory requirements and best practices.
What role do clinical trial management services play in compliance with the MDR?
Clinical trial management services ensure adherence to the MDR and improve the overall efficiency of the research process, as emphasized by specialists in the sector.




