Introduction
The shelf life of medical devices is a pivotal factor that influences not only their operational effectiveness but also patient safety and regulatory compliance. This article delves into the multifaceted aspects of shelf life, encompassing its:
- Definition
- Significance
- Rigorous testing protocols that ensure devices remain safe and effective throughout their intended duration
With an emphasis on the regulatory frameworks established by authorities such as Colombia's National Food and Drug Surveillance Institute (INVIMA), the discussion highlights the critical role of compliance in the medical device industry. Furthermore, the article examines the various factors that affect shelf life, the challenges manufacturers face in extending it, and the best practices that can be adopted to navigate this complex landscape. By understanding these elements, stakeholders can better ensure the reliability and longevity of medical products, ultimately safeguarding patient health and enhancing marketability.
Defining Shelf Life: Importance and Implications for Medical Devices
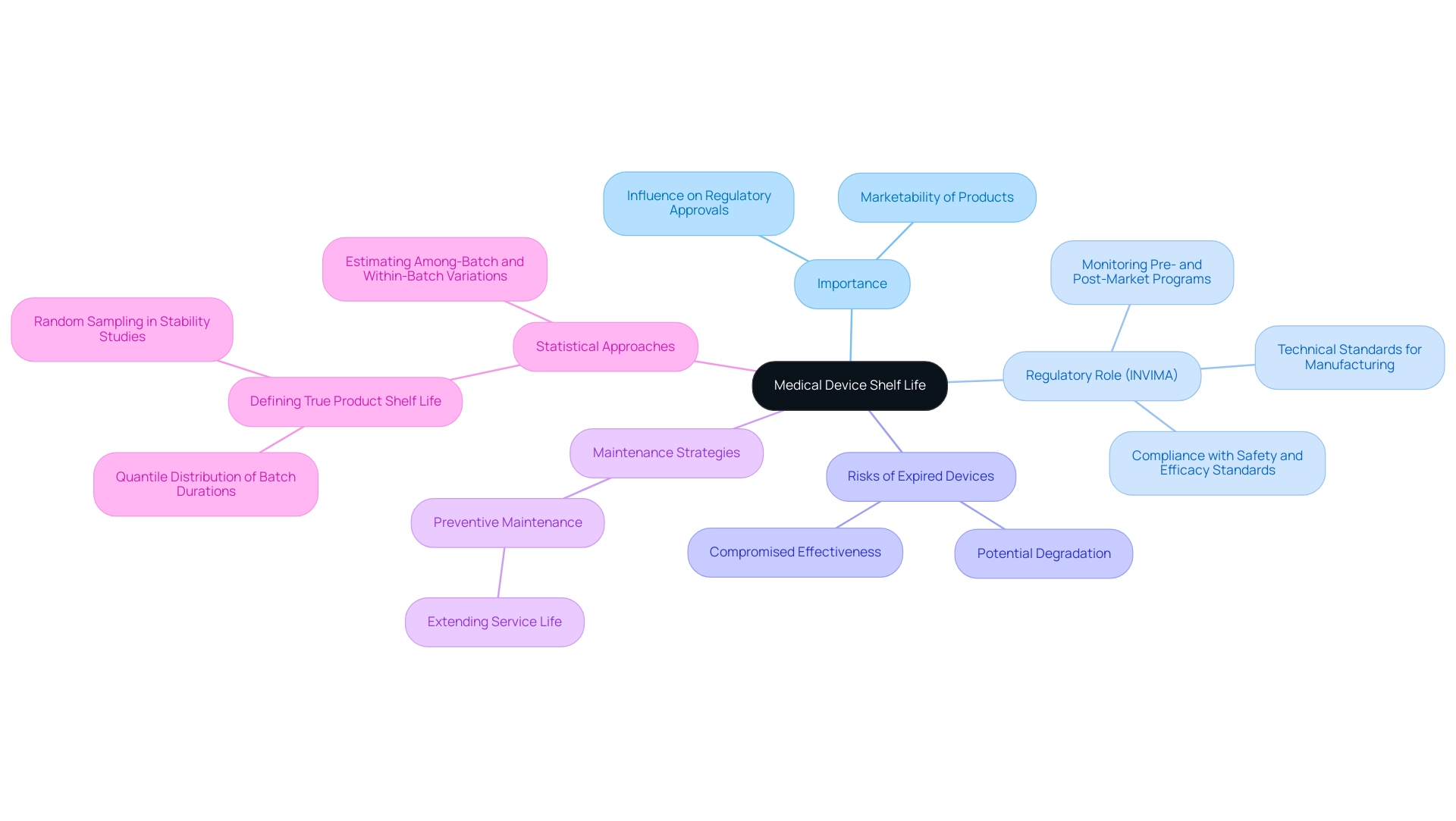
Shelf duration is a crucial factor in the medical device shelf life of healthcare instruments, outlining the period during which these products are anticipated to uphold safety and effectiveness under designated storage conditions. The significance of medical device shelf life cannot be overstated; it directly influences regulatory approvals and the overall marketability of healthcare products. In Colombia, the National Food and Drug Surveillance Institute (INVIMA) plays a pivotal role in regulating these aspects, ensuring that medical instruments comply with safety and efficacy standards.
INVIMA is responsible for monitoring both pre- and post-market programs, providing a comprehensive oversight framework that includes suggesting technical standards for the manufacturing, marketing, surveillance, and quality assurance of products. Devices that surpass their defined medical device shelf life may jeopardize patient safety, as they can incur potential degradation that compromises their effectiveness. This risk underscores the need for manufacturers to engage in rigorous testing and compliance protocols to uphold the medical device shelf life and integrity throughout its lifecycle, adhering to INVIMA's stringent regulatory framework.
Statistics suggest that preventive maintenance can considerably prolong the anticipated service duration of repairable products, reinforcing the necessity for manufacturers to be vigilant in their quality assurance processes. Moreover, the Working Group recommends defining actual product duration as a quantile of the distribution of true batch durations, ensuring a low likelihood of nonconforming batches at expiration. This modern viewpoint highlights the changing comprehension of storage duration factors, acknowledged by regulatory bodies like INVIMA, designated as a Level 4 health authority by PAHO/WHO.
Additionally, an alternative strategy proposed by the Working Group treats batches in stability studies as a random sample, allowing for separate estimation of among-batch and within-batch variations, which provides a more accurate estimate of true product shelf life, particularly in relation to medical device shelf life. As mentioned by Plastic Ingenuity, 'Our innovative, scalable healthcare solutions enable you to bring drug therapies, equipment, and diagnostic products to the market quicker.' This emphasizes the equilibrium between speed and safety in health product development, particularly under the regulatory oversight of INVIMA.
Comprehending the implications of product longevity is crucial not only for regulatory compliance but also for protecting patient health and ensuring the reliability of healthcare products.
Testing Protocols for Determining Medical Device Shelf Life
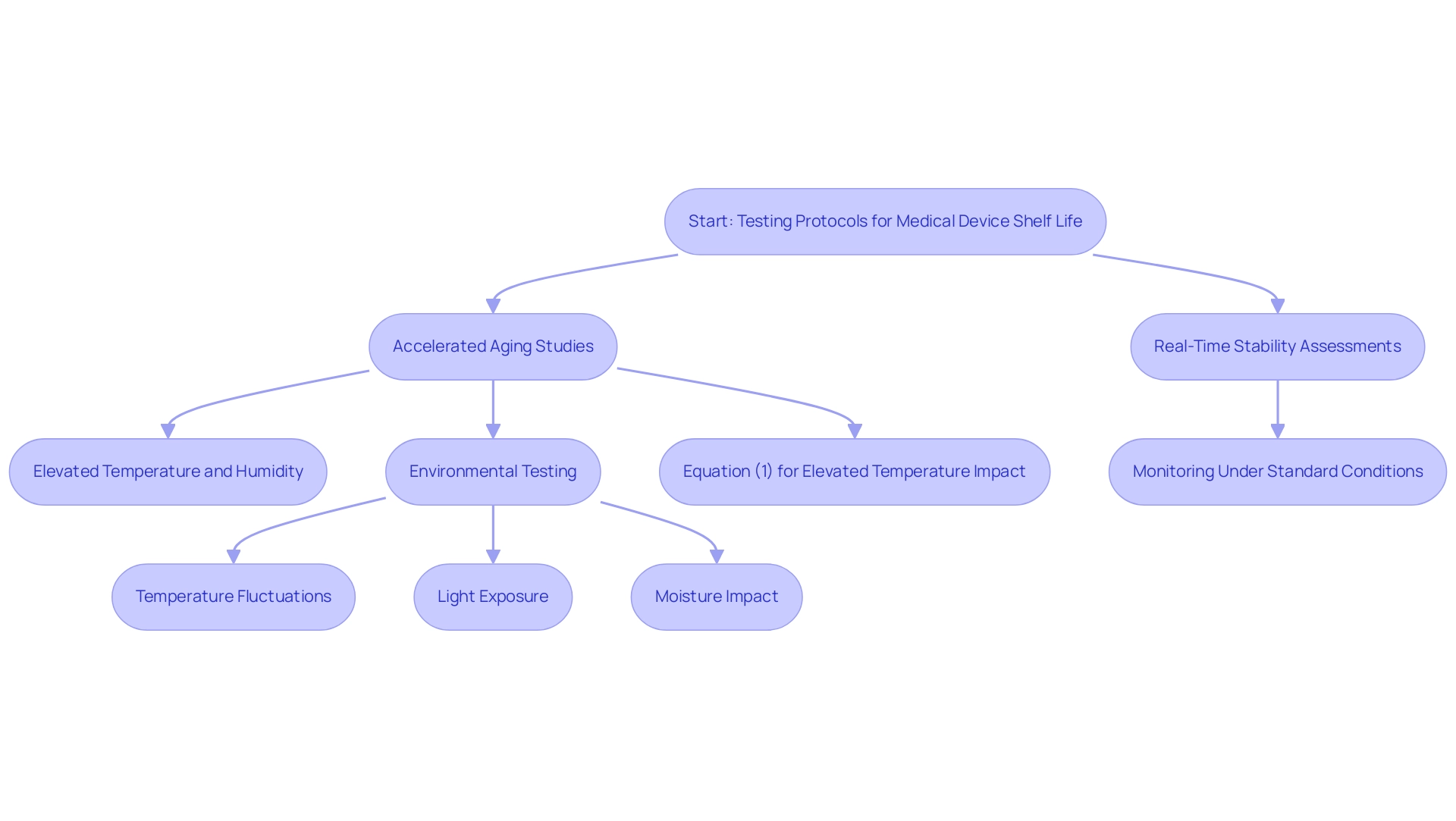
Testing protocols for determining the medical device shelf life predominantly rely on accelerated aging studies. These studies involve exposing equipment to elevated temperatures and humidity levels to replicate long-term storage conditions effectively. Notably, research has shown that aging can occur in a controlled environment, such as a reaction vessel with 5 bar pressure of oxygen, which enhances the relevance of these findings.
Complementing accelerated aging studies, real-time stability assessments are conducted, where items are monitored under standard storage conditions over an extended period. This dual approach not only provides comprehensive insights into the medical device shelf life but also incorporates environmental testing, evaluating how elements like temperature fluctuations, light exposure, and moisture impact device integrity. Moreover, Equation (1) is occasionally utilized to evaluate the effect of increased temperature on aging, further reinforcing the approaches applied in durability testing.
The integration of these methodologies is essential for establishing a strong durability assertion, ensuring adherence to strict industry standards. A relevant case study titled 'Application of Elevated Temperature in Medical Device Testing' outlines a method for predicting how aging affects materials that may be implanted in the human body, establishing a reference temperature of 37 °C (body temperature) and demonstrating how maintaining materials at higher temperatures can equate to extended aging periods. As Karl J. Hemmerich, General Manager and Corporate Technical Advisor at Isomedix Corp., observes,
Results will generally be conservative—that is, reduced durability will be achieved compared with that obtained via real-time aging.
This emphasizes the importance of employing both accelerated and real-time studies to offer a comprehensive understanding of a product's long-term performance.
Navigating Regulatory Compliance for Medical Device Shelf Life
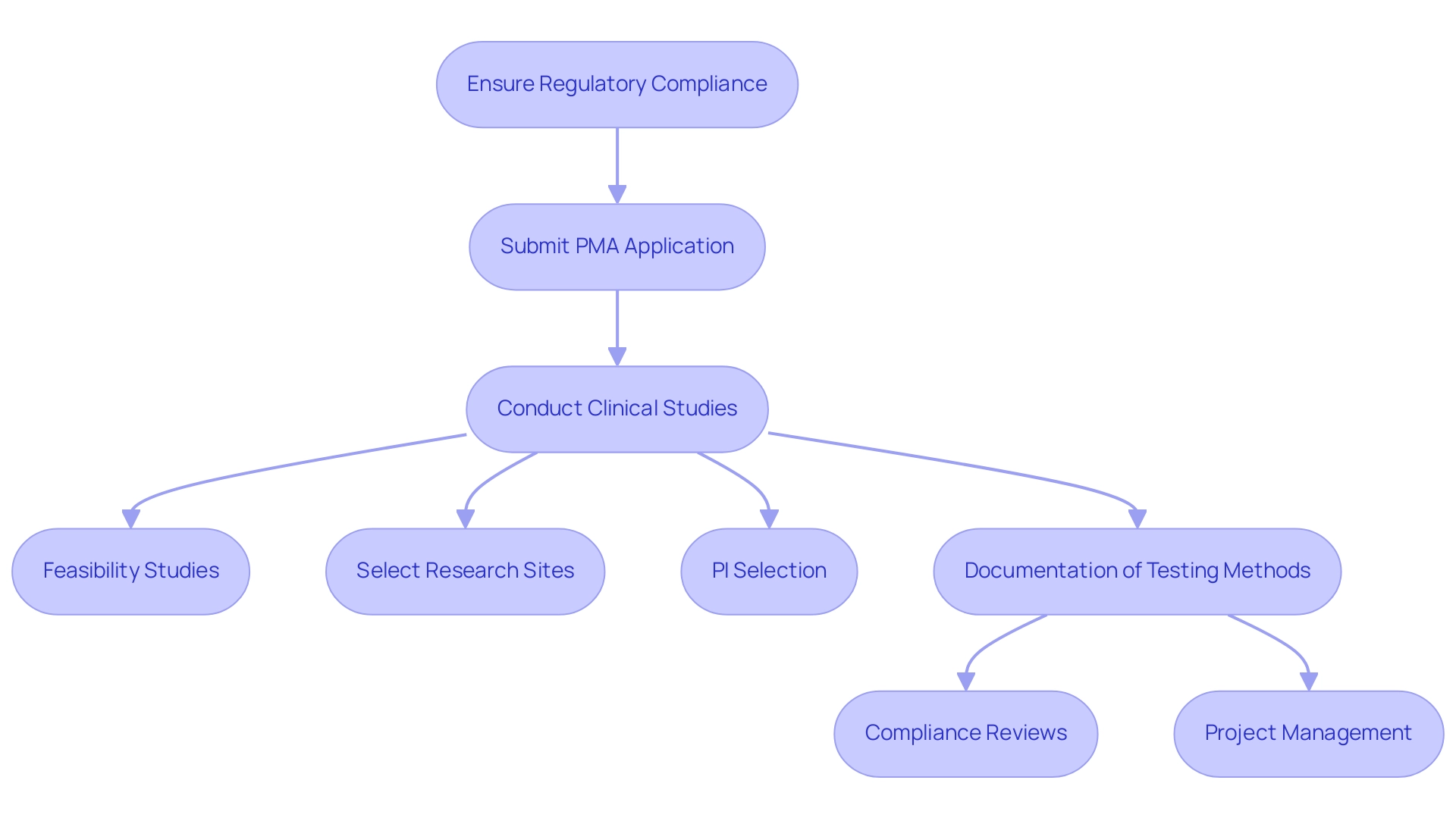
Ensuring regulatory compliance is essential for maintaining medical device shelf life and requires following established protocols from organizations like the FDA and Health Canada. Producers of Class III products must submit a premarket approval (PMA) application that includes results from clinical studies, demonstrating that the medical device shelf life supports the maintenance of safety and effectiveness throughout their intended duration. The FDA's incorporation of risk management into ISO 13485 and the Quality Management System Regulation (QMSR) improves the capacity to address patient and user needs while promoting access to quality products alongside scientific progress.
Our comprehensive clinical trial management services include:
- Feasibility studies
- Selection of research sites
- Principal investigator (PI) selection
- Thorough documentation of testing methods, results, and any deviations from established protocols
Led by experts like Ana Criado, Director of Regulatory Affairs, and Katherine Ruiz, who specializes in Regulatory Affairs for Medical Devices and In Vitro Diagnostics, we ensure compliance reviews and effective project management. Recent changes in regulatory definitions within the QMSR reflect the FDA's commitment to clearer guidance, aiding manufacturers in navigating the complex regulatory landscape, minimizing potential delays in market access, and addressing challenges such as regulatory hurdles and recruitment issues.
For more information on how we can support your clinical trials, BOOK A MEETING today.
Key Factors Affecting Medical Device Shelf Life
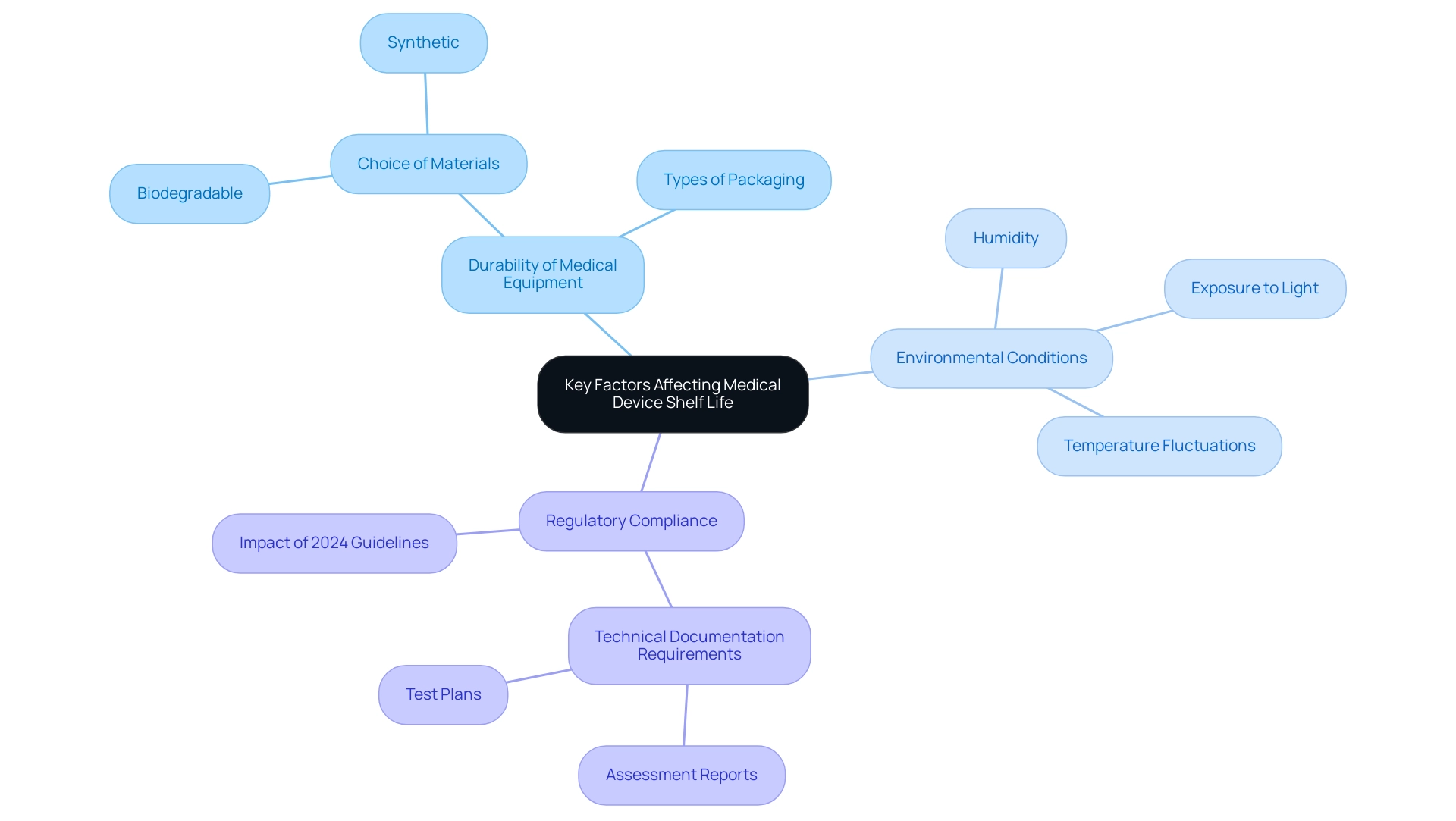
The durability of medical equipment directly impacts the medical device shelf life, which is affected by various factors such as the choice of materials, types of packaging, and environmental conditions during storage. Significantly, biodegradable materials frequently have reduced durations of usability compared to their synthetic counterparts. Furthermore, exposure to elements such as light, humidity, and temperature fluctuations can significantly accelerate degradation processes.
As the 2024 guideline revisions indicate, with 49 Class III devices being affected, manufacturers must meticulously consider these variables throughout the design and production phases. Furthermore, registration applicants are now obligated to submit various technical documents related to the medical device shelf life, including assessment reports and test plans, which are essential for ensuring compliance with regulatory expectations. This proactive method not only helps in enhancing the medical device shelf life but also adapts to the changing regulatory environment.
For example, a gadget may have a usable duration of four years but could cease to be effective within one year of opening, emphasizing the variability in anticipated durations based on product traits. As Dr. Duane Steffey, Principal Scientist at Exponent, points out,
Exponent's statisticians are experts in quantitative science and understand how the right assumptions and data analysis methods can come together to solve complex problems.
Such insights highlight the significance of systematic planning in the lifecycle management of healthcare products, ultimately affecting their reliability and longevity in clinical environments.
Challenges and Best Practices in Extending Medical Device Shelf Life
Extending the medical device shelf life of healthcare instruments involves navigating a complex landscape of challenges, particularly concerning material degradation and regulatory scrutiny from authorities such as INVIMA (Colombia National Food and Drug Surveillance Institute). Established in 1992, INVIMA plays a vital role in overseeing the marketing and manufacturing of health products, ensuring compliance with safety and quality standards. The Directorate for Healthcare Instruments and other Technologies within INVIMA specifically oversees and regulates healthcare instruments, proposing technical standards for their production and promotion.
With around 500,000 equipment models authorized by the FDA from about 23,000 producers, the essential requirement for thorough oversight of longevity cannot be emphasized enough. Manufacturers should prioritize comprehensive stability studies early in the development phase to identify potential degradation issues before they impact product integrity. A Quality Management System (QMS) aligned with international standards is crucial to manage various requirements for healthcare product longevity and to ensure compliance with regulatory expectations.
Furthermore, utilizing advanced packaging solutions plays a vital role in mitigating environmental effects that could undermine product quality. Engaging with regulatory bodies like INVIMA from the outset is crucial for aligning with compliance requirements. As specialists like Katherine Ruiz and Ana Criado highlight, the changing environment of equipment regulation requires that producers stay proactive in their quality control procedures to ensure that every element of production meets the highest standards.
Regular evaluations of medical device shelf life data are suggested to adjust to new discoveries, ensuring ongoing preservation of equipment integrity while tackling the latest regulatory challenges in a dynamic global environment. Furthermore, INVIMA's classification as a Level 4 health authority by PAHO/WHO underscores its competence and efficiency in fulfilling health regulation functions, further solidifying its role in ensuring the safety, efficacy, and quality of medical devices in Colombia.
Conclusion
Understanding the shelf life of medical devices is essential for ensuring their safety, efficacy, and regulatory compliance. This article has explored the definition of shelf life, its significance in the medical device industry, and the rigorous testing protocols that are crucial for validating the longevity of these products. The involvement of regulatory authorities, particularly Colombia's INVIMA, highlights the importance of adhering to established standards to protect patient health and maintain market integrity.
Key factors influencing shelf life, such as:
- Material selection
- Packaging
- Environmental storage conditions
were discussed, emphasizing the need for manufacturers to remain vigilant in their quality assurance processes. Challenges in extending shelf life, including:
- Material degradation
- Complexities of regulatory scrutiny
underscore the necessity for comprehensive stability studies and advanced packaging solutions. Best practices, including:
- Early engagement with regulatory bodies
- Regular reviews of shelf life data
are vital for navigating this intricate landscape.
Ultimately, a thorough understanding of shelf life implications not only enhances compliance with regulatory frameworks but also fosters the reliability and marketability of medical devices. By prioritizing these factors, stakeholders can contribute to safeguarding patient health while ensuring the long-term success of their products in the marketplace. The commitment to maintaining high standards in shelf life management will be paramount as the medical device industry continues to evolve and adapt to new challenges.
Frequently Asked Questions
What is shelf duration in the context of medical devices?
Shelf duration refers to the period during which medical devices are expected to maintain safety and effectiveness under specified storage conditions.
Why is the shelf life of medical devices important?
The shelf life of medical devices is crucial as it directly affects regulatory approvals and the marketability of healthcare products.
What role does INVIMA play in regulating medical device shelf life in Colombia?
INVIMA, the National Food and Drug Surveillance Institute, regulates the safety and efficacy of medical devices, ensuring compliance with standards and overseeing both pre- and post-market programs.
What risks are associated with medical devices that exceed their shelf life?
Medical devices that surpass their defined shelf life may pose risks to patient safety due to potential degradation that compromises their effectiveness.
How can manufacturers ensure compliance with shelf life regulations?
Manufacturers must engage in rigorous testing and compliance protocols to uphold medical device shelf life and integrity, adhering to INVIMA's regulatory framework.
What is the significance of preventive maintenance in relation to medical devices?
Preventive maintenance can significantly extend the anticipated service duration of repairable medical devices, highlighting the importance of quality assurance processes.
What modern approach is recommended for defining product duration?
The Working Group recommends defining actual product duration as a quantile of the distribution of true batch durations to minimize the likelihood of nonconforming batches at expiration.
What methodologies are used to determine medical device shelf life?
Testing protocols primarily rely on accelerated aging studies and real-time stability assessments to evaluate the long-term performance of medical devices.
How do accelerated aging studies work?
Accelerated aging studies expose medical devices to elevated temperatures and humidity levels to replicate long-term storage conditions, providing insights into their durability.
What is the purpose of real-time stability assessments?
Real-time stability assessments monitor medical devices under standard storage conditions over time, evaluating the impact of environmental factors on device integrity.
Why is it important to use both accelerated aging studies and real-time assessments?
Using both methodologies provides a comprehensive understanding of a product's long-term performance and ensures adherence to strict industry standards.




