Introduction
In the realm of healthcare, the sterilization of medical devices is a fundamental practice that ensures the safety and efficacy of treatments. With a significant portion of the adult population managing chronic diseases, the demand for reliable sterilization methods has never been more critical. This article delves into the various sterilization techniques employed in the medical device industry, exploring their unique advantages and limitations.
- From traditional methods like steam sterilization
- To emerging technologies such as vaporized hydrogen peroxide, each approach plays a vital role in maintaining high standards of patient safety.
Additionally, the discussion includes the regulatory landscape that governs these practices, highlighting the importance of compliance in an ever-evolving healthcare environment. As the industry seeks innovative solutions to address safety concerns, understanding these sterilization methods becomes imperative for manufacturers and healthcare providers alike.
Exploring Common Medical Device Sterilization Methods
Medical device cleansing plays a pivotal role in safeguarding the integrity and safety of healthcare products, especially considering that, according to a report by the National Association of Chronic Diseases Directors published in April 2022, 60% of adult Americans have at least one chronic disease, emphasizing the critical need for effective cleansing practices. Various sterilization techniques are employed, each with distinct applications and effectiveness:
- Steam Sterilization (Autoclaving): This method harnesses high-pressure steam to eradicate bacteria and spores, making it highly effective for heat-resistant items. While widely utilized, it may not be suitable for all medical equipment due to temperature sensitivities.
- Ethylene Oxide (EtO) Sterilization: As a gas, EtO effectively penetrates packaging and devices, rendering it an optimal choice for heat-sensitive materials. However, it demands stringent handling protocols due to its inherent toxicity. A recent guidance issued by the FDA on November 26, 2024, streamlined processes for manufacturers in response to supply chain disruptions, allowing certain changes in sanitation facilities without prior approval. This guidance reflects the ongoing evolution of disinfection regulations, which includes a regulatory review of the Occupational Safety and Health Administration's Ethylene Oxide Standard conducted in 2005.
- Radiation Sterilization: Utilizing gamma rays or electron beams, this approach excels at removing microorganisms and is particularly beneficial for single-use healthcare products, as it can penetrate packaging effectively.
- Vaporized Hydrogen Peroxide: A more modern technique, this process utilizes vaporized hydrogen peroxide to disinfect instruments without the negative impacts linked to EtO.
Grasping these medical device sterilization methods is crucial for choosing the most appropriate option based on the specific needs of various healthcare instruments. With North America projected to hold a 35.43% share of the equipment market in 2024, the emphasis on effective decontamination methods continues to grow, reflecting the industry's commitment to maintaining high safety standards. The implications of these practices are significant, particularly as the healthcare landscape adapts to meet the needs of a population with a high prevalence of chronic diseases.
The Role of Ethylene Oxide in Medical Device Sterilization: Benefits and Concerns
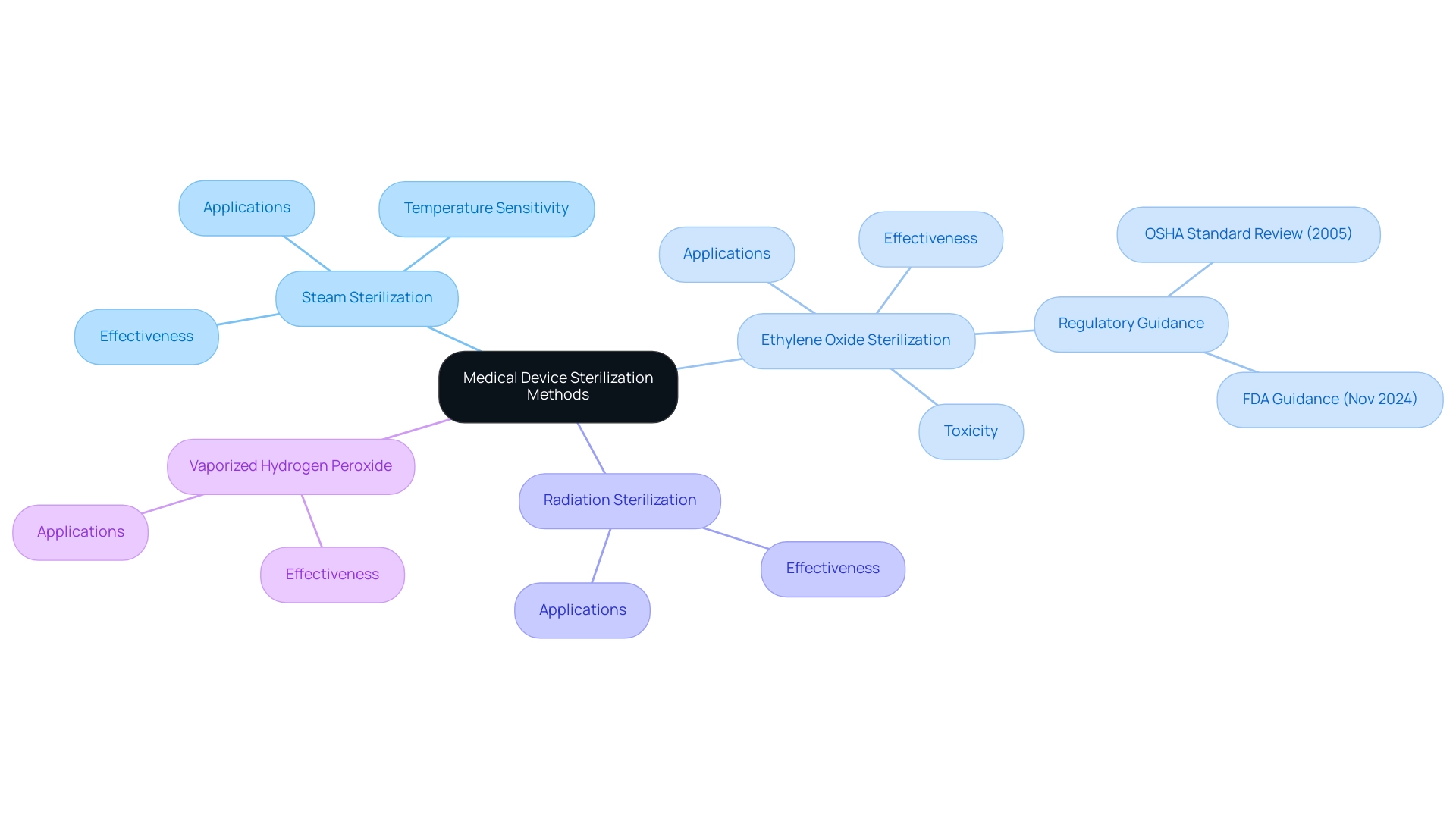
Ethylene Oxide (EtO) is an essential agent for medical device sterilization methods in the healthcare equipment sector, particularly valued for its efficacy at low temperatures, which allows for the safe cleaning of heat-sensitive instruments. The key advantages of EtO processing include:
- Broad Compatibility: EtO is capable of disinfecting complex devices with intricate designs, making it an invaluable option for a wide range of medical applications. This low-temperature process, as one of the medical device sterilization methods, is particularly advantageous for materials that cannot endure high heat, ensuring the integrity of sensitive components.
- Aeration Process: After the disinfection procedure, aeration usually requires around 12 hours, which is a significant factor for the overall timeline of EtO treatment.
Despite these benefits, several concerns surrounding EtO warrant attention:
- Toxicity: Classified as a known carcinogen, Ethylene Oxide has drawn significant regulatory scrutiny, leading to discussions surrounding exposure limits and workplace safety. Dr. Amanda Sivek, a principal project engineer at the ECRI Institute, emphasizes the gravity of this issue, stating,
Currently, there are no validated industrial methods for disinfection that could completely replace EtO processes, so additional closures of EtO processing facilities would have the potential to impair the U.S. healthcare system.
- Environmental Impact: The use of EtO raises critical questions regarding potential environmental risks. Recent findings from Phase I of a study indicated the lowest measured 24-hour EtO concentration was 0.42 µg/m³, suggesting that annual average ambient air concentrations could be approximately 200 times higher than the Initial Risk Screening level right outside disinfection facilities. This concern is compounded by the EPA's cancer risk evaluations for Ethylene Oxide, which have been criticized for lacking scientific justification. Independent analyses indicate that the actual risk of Ethylene Oxide exposure may be significantly lower than the EPA's conclusions, contradicting observed health studies. Moreover, companies collaborating with Ethylene Oxide are actively investing in research and product stewardship technologies to mitigate these risks while ensuring the ongoing effectiveness of disinfection processes. Thus, the discussion regarding medical device sterilization methods not only emphasizes their essential role in the sanitation of healthcare instruments but also stresses the significance of continuous research and product management in addressing the issues related to their application.
Alternatives to Ethylene Oxide: Emerging Sterilization Technologies
In response to the increasing demand for safer disinfection techniques, significant alternatives to Ethylene Oxide (EtO) have gained popularity as medical device sterilization methods in the healthcare equipment sector.
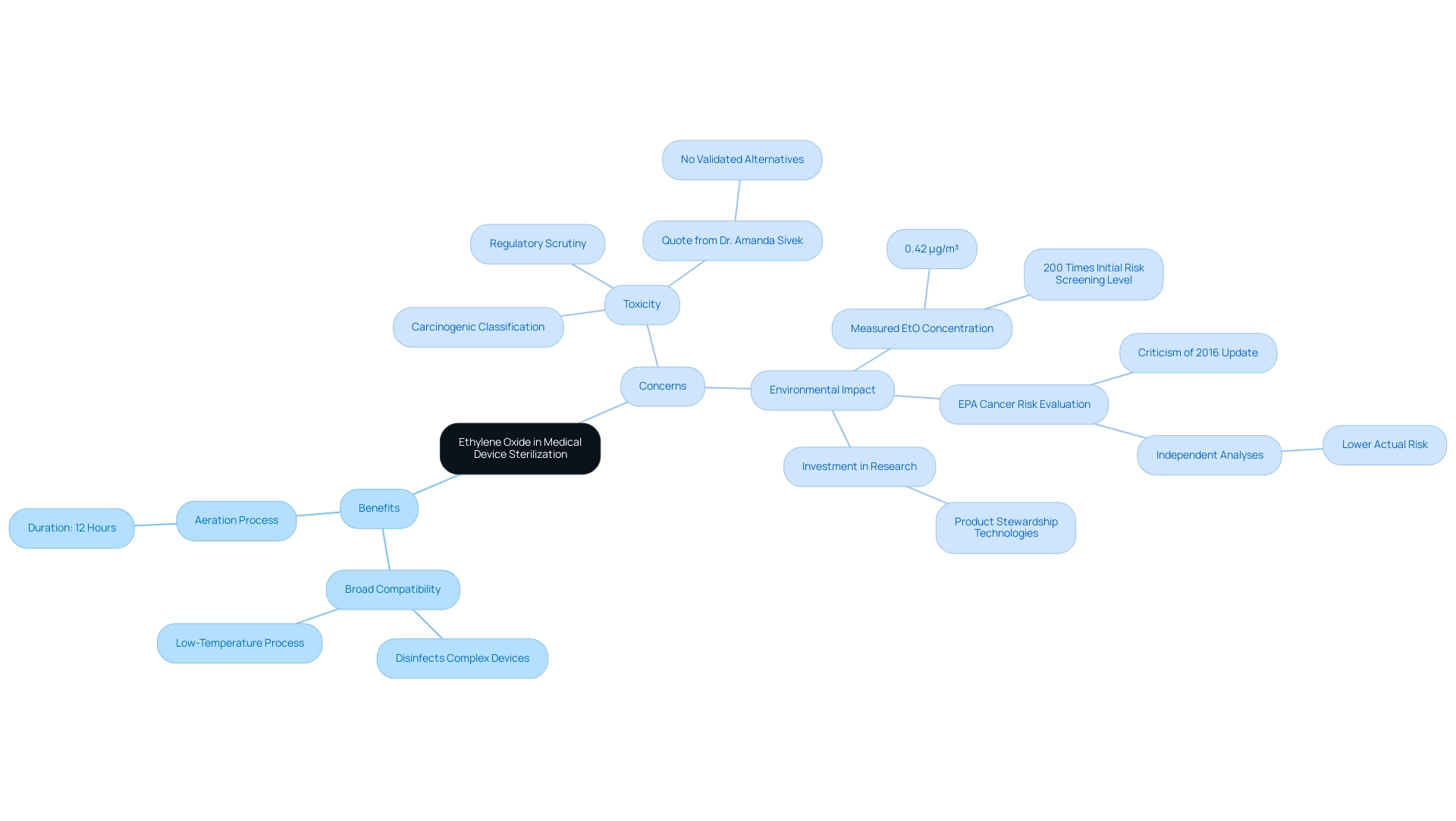
- Vaporized Hydrogen Peroxide (VHP) stands out for its effectiveness and reduced toxicity, making it an ideal choice for a diverse range of medical devices.
Its application has been supported by emerging studies demonstrating its ability to achieve high log reductions of microorganisms, which is crucial for ensuring patient safety. According to industry reports, the effectiveness of VHP is anticipated to play a major role in the projected market growth of USD 3.38 billion by 2034 in the Asia Pacific region, where enhancements in healthcare infrastructure are boosting demand for safer medical device sterilization methods.
-
Nitrogen Dioxide (NO2) is an innovative disinfection technology that shows considerable promise for heat-sensitive materials.
- This method provides quick cleaning cycles, which is especially advantageous in fast-paced clinical environments where time efficiency is crucial.
- Experts from Steris Corporation have noted that NO2 can greatly reduce turnaround times in disinfection processes, enhancing operational efficiency.
-
Steam with Hydrogen Peroxide represents a synergistic approach, combining the established efficacy of steam disinfection with the advantages of hydrogen peroxide.
- This method not only improves disinfection effectiveness but also contributes to a reduced environmental impact, aligning with the industry's growing emphasis on sustainability.
- According to 3M Company, combining hydrogen peroxide with steam sanitization methods is a progression in tackling both effectiveness and environmental issues.
These medical device sterilization methods address the safety issues related to EtO while complying with changing regulatory requirements, thus preparing the healthcare sector for improved adherence and innovation.
Navigating Regulatory Challenges in Medical Device Sterilization
Navigating the regulatory landscape for medical device sterilization methods necessitates a thorough understanding of evolving standards and guidelines. Key considerations include medical device sterilization methods.
- FDA Guidelines: It is imperative for manufacturers to stay abreast of the most recent FDA requirements for validation of sterilizing procedures. This encompasses the essential requirement to showcase the effectiveness and safety of the selected medical device sterilization methods. As mentioned by FDA Commissioner Scott Gottlieb, M.D., the Agency is actively taking measures to prevent possible shortages of healthcare instruments while ensuring safe and effective sanitization practices, especially after the closure of a major contract sanitizing facility. Significantly, approximately 50% of all sterile healthcare instruments in the U.S. are sanitized using ethylene oxide, highlighting the importance of medical device sterilization methods for efficient disinfection techniques.
- Following international standards, like ISO 11135, which specifically regulates ethylene oxide (EtO) decontamination, is crucial for maintaining compliance and ensuring global market access for medical device sterilization methods. Compliance with these standards not only enhances product safety but also aligns with regulatory expectations regarding medical device sterilization methods worldwide.
- The regulatory review of the Occupational Safety and Health Administration's Ethylene Oxide Standard conducted in 2005 remains relevant, underscoring the ongoing importance of medical device sterilization methods in ensuring safe practices.
- Post-Market Surveillance: Manufacturers carry the ongoing responsibility of monitoring the safety and effectiveness of sterilized devices and the medical device sterilization methods employed once they reach the market. This includes a proactive approach in responding to any adverse events related to disinfection processes. With the FDA's guidance released on November 26, 2024, concerning changes in ethylene oxide decontamination activities, it is essential for manufacturers to stay alert about regulatory shifts related to medical device sterilization methods and to engage actively with regulatory bodies to ensure successful decontamination practices. Additionally, insights from experts like Ana Criado, who has extensive experience with INVIMA and is well-versed in Regulatory Affairs, biomedical engineering, and health economics, underscore the importance of understanding these regulations. Her expertise also encompasses the developing cannabis sector in Colombia, which may affect sanitation standards for cannabis-based healthcare products.

Choosing the Right Sterilization Method for Medical Devices
Choosing a suitable disinfection technique for medical instruments is a crucial procedure that depends on several key factors:
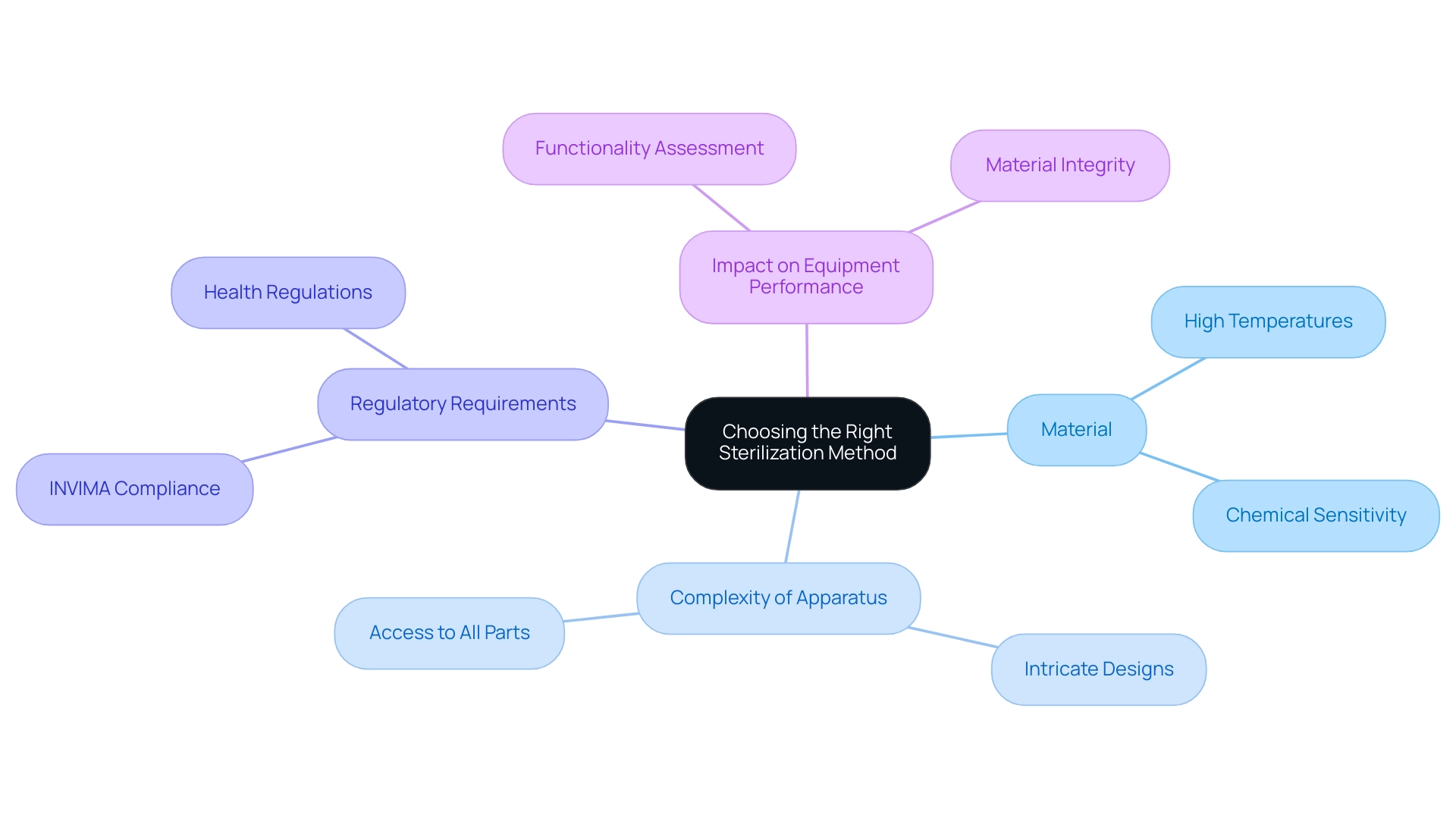
- Material: It is essential to evaluate whether the item's material can withstand high temperatures or is sensitive to chemical agents. Comprehending the material properties is essential for selecting suitable medical device sterilization methods.
- Complexity of the Apparatus: The design intricacies of the apparatus must be taken into account. It is essential to confirm that the selected procedure can effectively reach all parts of the apparatus, particularly in intricate shapes that may conceal impurities.
- Regulatory Requirements: Compliance with INVIMA guidelines and applicable regulations is non-negotiable. As a Level 4 regional reference health authority, INVIMA guarantees that techniques for sanitization comply with relevant standards. INVIMA's specific responsibilities include monitoring the manufacturing processes, conducting inspections, and evaluating compliance with health regulations, which ultimately safeguard patient safety and ensure legal compliance in Colombia.
- Impact on Equipment Performance: A comprehensive assessment of how the sanitization process might influence the functionality or safety of the apparatus is essential. Specific techniques may change material properties or undermine equipment integrity, which could have substantial consequences in clinical environments. Failure to comply with INVIMA regulations can result in severe repercussions, such as product recalls and legal responsibilities, emphasizing the significance of following these guidelines.
By carefully evaluating these aspects, manufacturers and clinical researchers can make informed choices about medical device sterilization methods, ultimately enhancing patient safety and ensuring the effectiveness of medical instruments. The significance of these considerations is highlighted by the insights from the National Center for Emerging and Zoonotic Infectious Diseases, which state, 'Factor only relevant for reused surgical/medical instruments.' This highlights that the selection of medical device sterilization methods is particularly crucial when dealing with reused devices, emphasizing the need for careful consideration.
Conclusion
The exploration of medical device sterilization methods reveals a complex landscape that is essential for ensuring patient safety and the efficacy of healthcare products. Traditional methods, such as steam sterilization and ethylene oxide, each present unique advantages and challenges. While steam sterilization is effective for heat-resistant items, ethylene oxide remains a critical option for heat-sensitive devices, albeit with significant regulatory scrutiny due to its toxicity and environmental impact.
Emerging technologies, including vaporized hydrogen peroxide and nitrogen dioxide, offer promising alternatives that address safety concerns associated with traditional methods. These innovations not only enhance sterilization effectiveness but also align with evolving regulatory expectations, reflecting the industry's commitment to adapting to the needs of a changing healthcare environment.
Navigating the regulatory framework is paramount for manufacturers, as compliance with FDA guidelines and international standards ensures the safe and effective sterilization of medical devices. The ongoing evolution of regulations underscores the importance of proactive engagement with regulatory bodies to maintain high safety standards.
In conclusion, understanding and selecting the appropriate sterilization method is critical for manufacturers and healthcare providers alike. By considering factors such as device material, complexity, and regulatory requirements, stakeholders can make informed decisions that ultimately enhance patient safety and the effectiveness of medical treatments. The commitment to advancing sterilization practices is vital, particularly as the healthcare landscape continues to evolve in response to the growing prevalence of chronic diseases.
Frequently Asked Questions
Why is medical device cleansing important?
Medical device cleansing is crucial for safeguarding the integrity and safety of healthcare products, especially given that 60% of adult Americans have at least one chronic disease, highlighting the need for effective cleansing practices.
What are the main sterilization techniques used for medical devices?
The main sterilization techniques include: 1. Steam Sterilization (Autoclaving): Uses high-pressure steam to eliminate bacteria and spores, effective for heat-resistant items but may not suit all equipment. 2. Ethylene Oxide (EtO) Sterilization: A gas that penetrates packaging and is ideal for heat-sensitive materials, but requires strict handling due to toxicity. 3. Radiation Sterilization: Employs gamma rays or electron beams, effective for single-use products as it penetrates packaging. 4. Vaporized Hydrogen Peroxide: A modern technique that disinfects instruments without the downsides of EtO.
What are the advantages of Ethylene Oxide (EtO) sterilization?
The advantages of EtO include: - Broad Compatibility: Effective for complex devices and materials that cannot withstand high heat. - Low Temperature: Ensures the integrity of sensitive components. - Aeration Process: Requires about 12 hours post-disinfection, which is important for treatment timelines.
What concerns are associated with Ethylene Oxide (EtO)?
Concerns regarding EtO include: - Toxicity: Classified as a carcinogen, leading to regulatory scrutiny and discussions about exposure limits and workplace safety. - Environmental Impact: Potential environmental risks from EtO emissions, with studies indicating higher air concentrations near disinfection facilities.
How does the current regulatory landscape affect EtO sterilization?
Recent FDA guidance has streamlined processes for manufacturers regarding sanitation facilities, reflecting ongoing regulatory evolution in response to supply chain disruptions and safety standards.
What is the significance of ongoing research and product stewardship in medical device sterilization?
Continuous research and product stewardship are vital for mitigating risks associated with EtO while ensuring the effectiveness of disinfection processes, addressing both health and environmental concerns.




