Overview
The article focuses on the evolving landscape of Medtech in Latin America and the various challenges and opportunities researchers face in clinical trials within this region. It highlights the importance of strategic collaborations, regulatory advantages, and emerging technologies that can enhance study execution and patient recruitment, thereby positioning Latin America as a promising hub for Medtech innovation.
Introduction
In recent years, Latin America has emerged as a dynamic player in the Medtech sector, showcasing a wave of innovation and clinical research that is reshaping the landscape of healthcare. With a growing number of startups focusing on medical devices and digital health technologies, the region is ripe with opportunities for clinical trials.
However, the journey is not without its hurdles; companies must navigate regulatory complexities, resource limitations, and cultural nuances. Strategic partnerships are becoming essential to overcome these challenges, as seen in initiatives aiming to position cities like Barranquilla as key hubs for clinical research.
As the demand for specialized medical technologies surges, understanding the intricacies of this evolving market is crucial for stakeholders eager to capitalize on Latin America's potential in the Medtech arena.
The Evolving Landscape of Medtech in Latin America
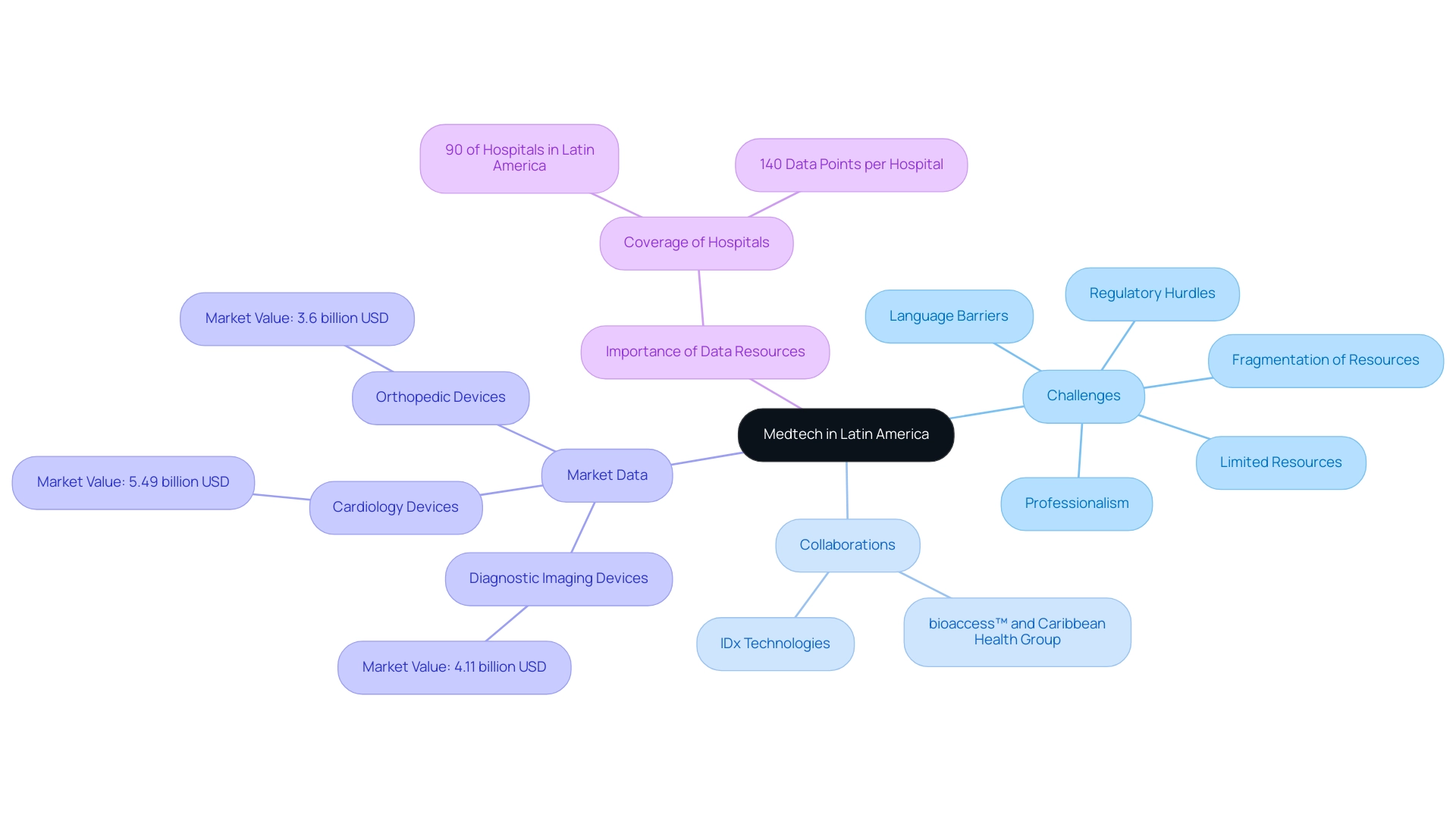
Latin America has established itself as a vibrant center for Medtech innovation and research, with a notable focus on Medtech in Latin American trials, characterized by a surge in startups dedicated to medical devices and digital health technologies. However, companies involved in Medtech in Latin American trials face multifaceted challenges, including:
- Regulatory hurdles
- Limited resources
- Professionalism
- Language barriers
- Fragmentation of resources
These challenges are being addressed through strategic collaborations.
Significantly, collaborations such as that of bioaccess™ and Caribbean Health Group seek to establish Barranquilla as a premier location for medical studies, backed by Colombia's Minister of Health. The region's varied healthcare system, particularly in Brazil, Mexico, and Argentina, offers distinct possibilities for research studies focused on Medtech in Latin American trials. The cardiology devices market alone generated approximately 5.49 billion USD, while the diagnostic imaging devices and orthopedic devices markets generated 4.11 billion USD and 3.6 billion USD, respectively.
These figures underscore a robust investment landscape that reflects the escalating demand for specialized medical technologies. Additionally, collaborations like IDx Technologies' partnership with bioaccess™ to identify ophthalmology centers in South America for AI-driven disease detection and GlobalCare Clinical Trials' improvements to ambulatory services in Colombia, which resulted in over 50% reduction in recruitment time and 95% retention rates, emphasize the progressive shift towards efficient study execution. As mentioned by Guillaume Corpart, CEO of Global Health Intelligence, 'We cover approximately 90% of all the hospitals in the southern continent within our database, with more than 140 data points for each.'
This extensive data resource is crucial for researchers seeking to understand the intricacies of the area's healthcare landscape, making it vital for investigators to comprehend the regional subtleties as they plan and execute studies. Moreover, there is an urgent need for a solution-driven approach to bridge the gap between innovation and execution, particularly in Medtech in Latin American trials, ensuring that the potential of the region's Medtech sector is fully realized.
Navigating the Regulatory Framework for Clinical Trials
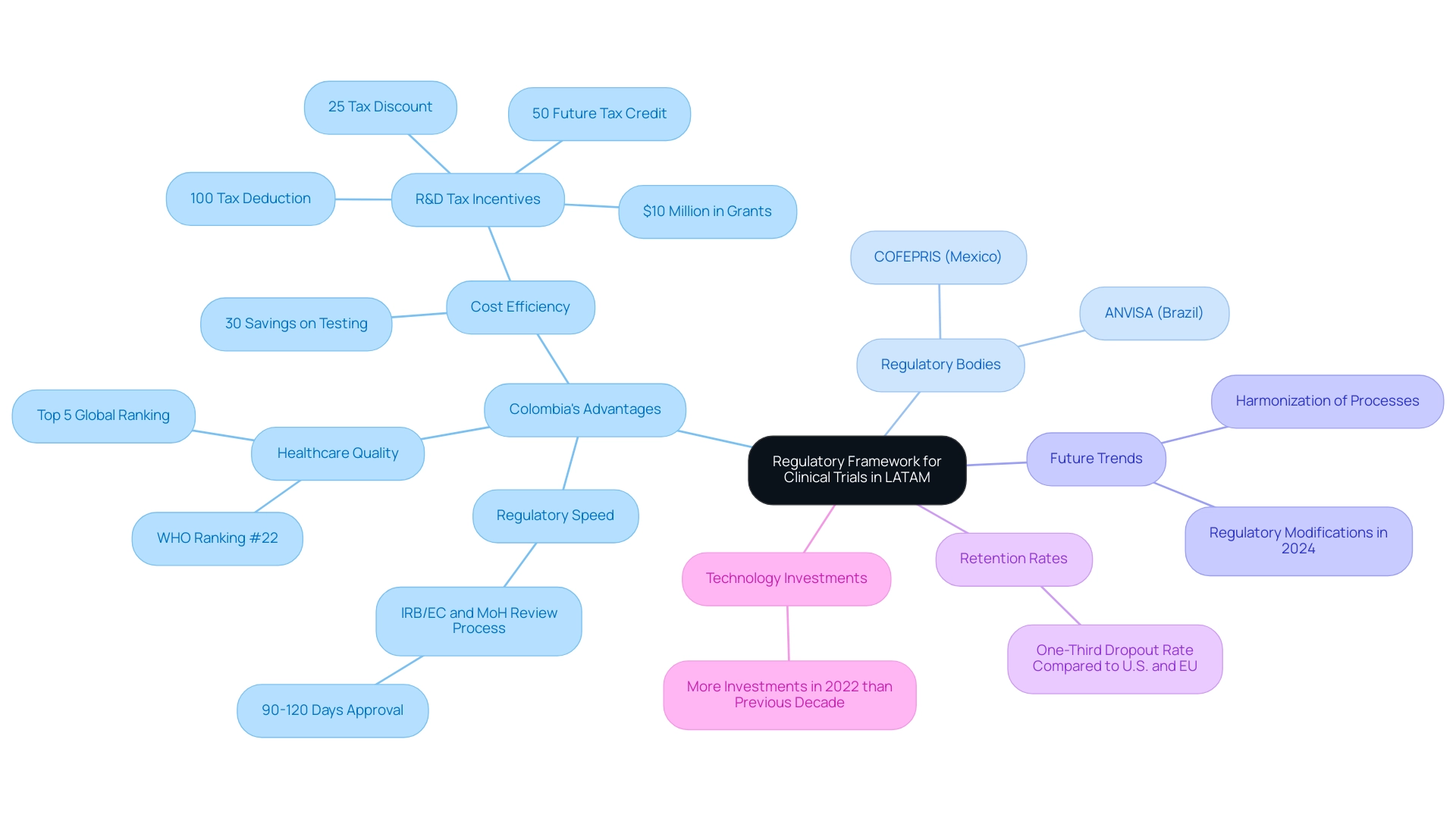
Navigating the regulatory environment for research studies in Latin regions presents unique challenges, primarily due to the differing regulations among various nations. However, Colombia stands out as a premier destination for first-in-human clinical studies, offering significant advantages in cost efficiency, regulatory speed, high-quality healthcare, and robust patient recruitment. The total IRB/EC and MoH (INVIMA) review process in Colombia takes just 90-120 days, allowing for quicker approvals compared to other regions.
Furthermore, the nation provides savings of over 30 percent on testing expenses compared to North and Western Europe, along with R&D tax incentives that encompass:
- A 100% tax deduction for investments in science and technology
- A 25% tax discount
- A 50% future tax credit
- Around $10 million in government grants
Colombia's healthcare system is ranked #22 by the World Health Organization among 191 nations, and its hospitals are acknowledged as some of the finest in South America by 'Economía' publication. Furthermore, the International Living publication ranks Colombia's healthcare system within the top five in the world.
Recent developments indicate a significant trend toward the harmonization of processes and expedited approval timelines, particularly in key markets like Brazil, Argentina, and Mexico. For example, the dropout rates in the region are roughly one-third of those observed in the U.S. and EU, indicating superior retention rates in studies, which can be linked to the area's enhanced regulatory system. Moreover, LATAM experienced more technology investments in 2022 than in the previous decade combined, further underscoring the region's growth potential for Medtech in Latin American trials.
Researchers should prioritize familiarizing themselves with local regulatory bodies, such as ANVISA in Brazil and COFEPRIS in Mexico, to ensure compliance with safety standards and ethical guidelines. Mariana Bei, Sr. Director of Clinical Operations and Brazil GMBA at Parexel, emphasizes this point:
Having a local presence and leadership that is based in the LATAM region strengthens the relationships that Parexel builds with local talent, regulatory authorities based in the area, local associations, and the global network of sites overall.
Understanding the role of ethics committees in the study approval process is also crucial for a smooth research experience.
The continuous regulatory modifications in the southern region, especially those expected in 2024, are poised to further simplify processes, offering substantial prospects for medical research and development in the area. By staying informed about these evolving regulations, including the rigorous ICH/GCP certification process that hospitals in Colombia must undergo, researchers can enhance their chances of successful study outcomes.
Advantages of Conducting Clinical Trials in Latin America
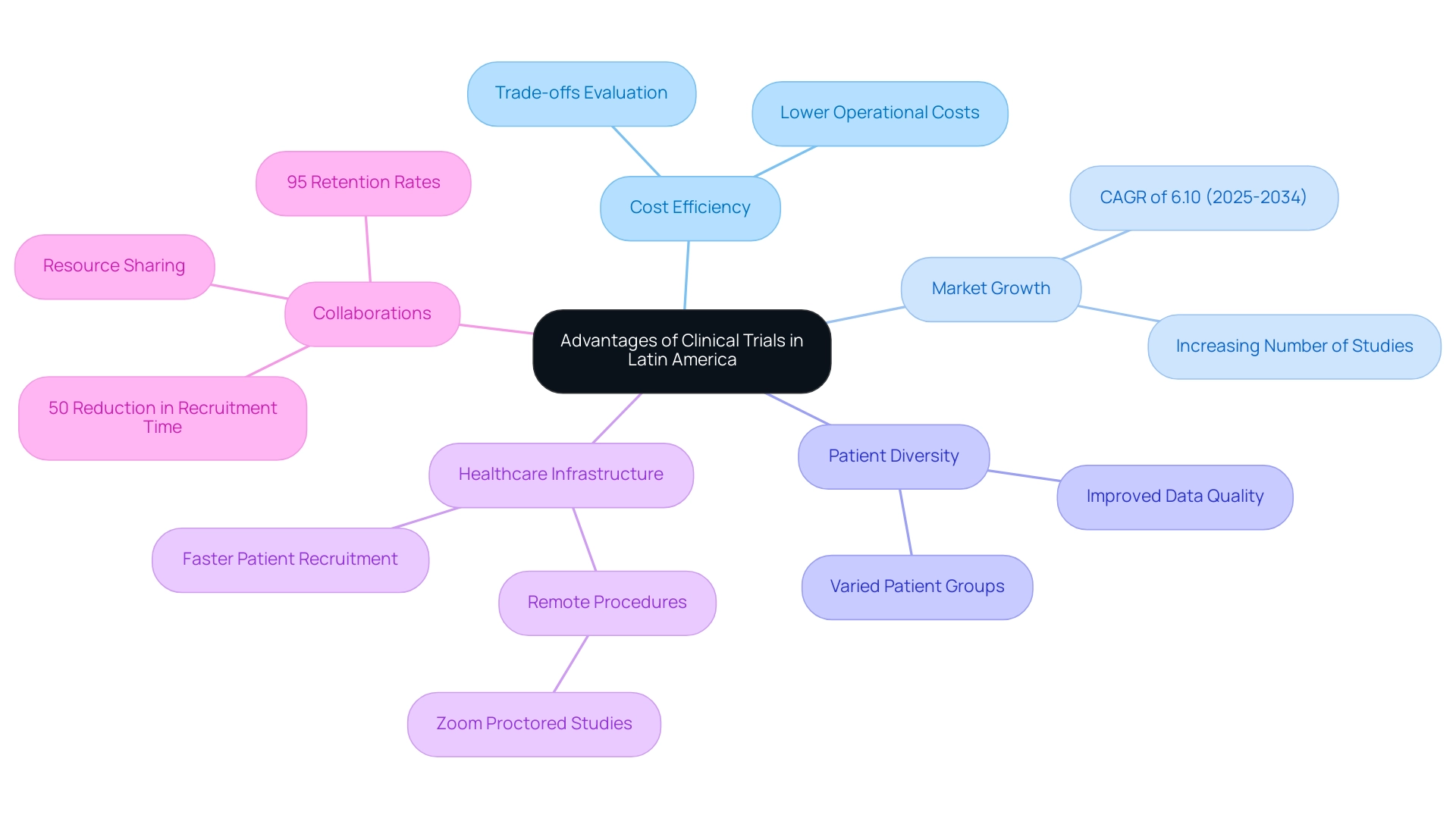
Carrying out research studies in Latin America offers numerous benefits, especially regarding cost efficiency, as operational costs can be significantly less than in North America and Europe. The area is undergoing a notable growth in the research studies market, expected to increase at a CAGR of 6.10% from 2025 to 2034. This expansion is fueled by the rising number of studies in developing areas and growing needs for advanced therapies, as demonstrated by ReGelTec's Early Feasibility Study on HYDRAFIL™ for chronic low back pain treatment in Colombia.
The procedures in this study were proctored remotely via Zoom, and all eleven patients were treated using a patented hydrogel injected into the nucleus of a degenerated disc via a 17-gauge needle. Moreover, joint initiatives, like those between bioaccess™ and Caribbean Health Group, are establishing Barranquilla as a prominent location for research in the region, with backing from the Colombian Minister of Health. Access to varied patient groups is another essential advantage; this diversity improves data quality and generalizability of study outcomes.
Moreover, the region's improving healthcare infrastructure facilitates faster patient recruitment and streamlines study execution, making it an attractive option for sponsors seeking efficiency while managing logistical, regulatory, and ethical considerations. The partnership between GlobalCare Clinical Trials and bioaccess™ further illustrates this landscape, achieving over a 50% reduction in recruitment time and 95% retention rates. The rising trend of collaboration among biopharmaceutical companies to share resources also helps mitigate the high costs associated with drug development.
As pointed out by Patricio Ledesma, Head of Clinical Operations at Sofpromed CRO, the rising prevalence of chronic illnesses and the expanding elderly population further emphasize the significance of carrying out research studies in Latin America, leading to a higher demand for innovative therapies. Moreover, these medical studies greatly influence local economies by generating employment, improving healthcare services, and promoting international cooperation, which further reinforces the area's role in Medtech in Latin American trials.
The Role of CROs in Advancing Medtech Research
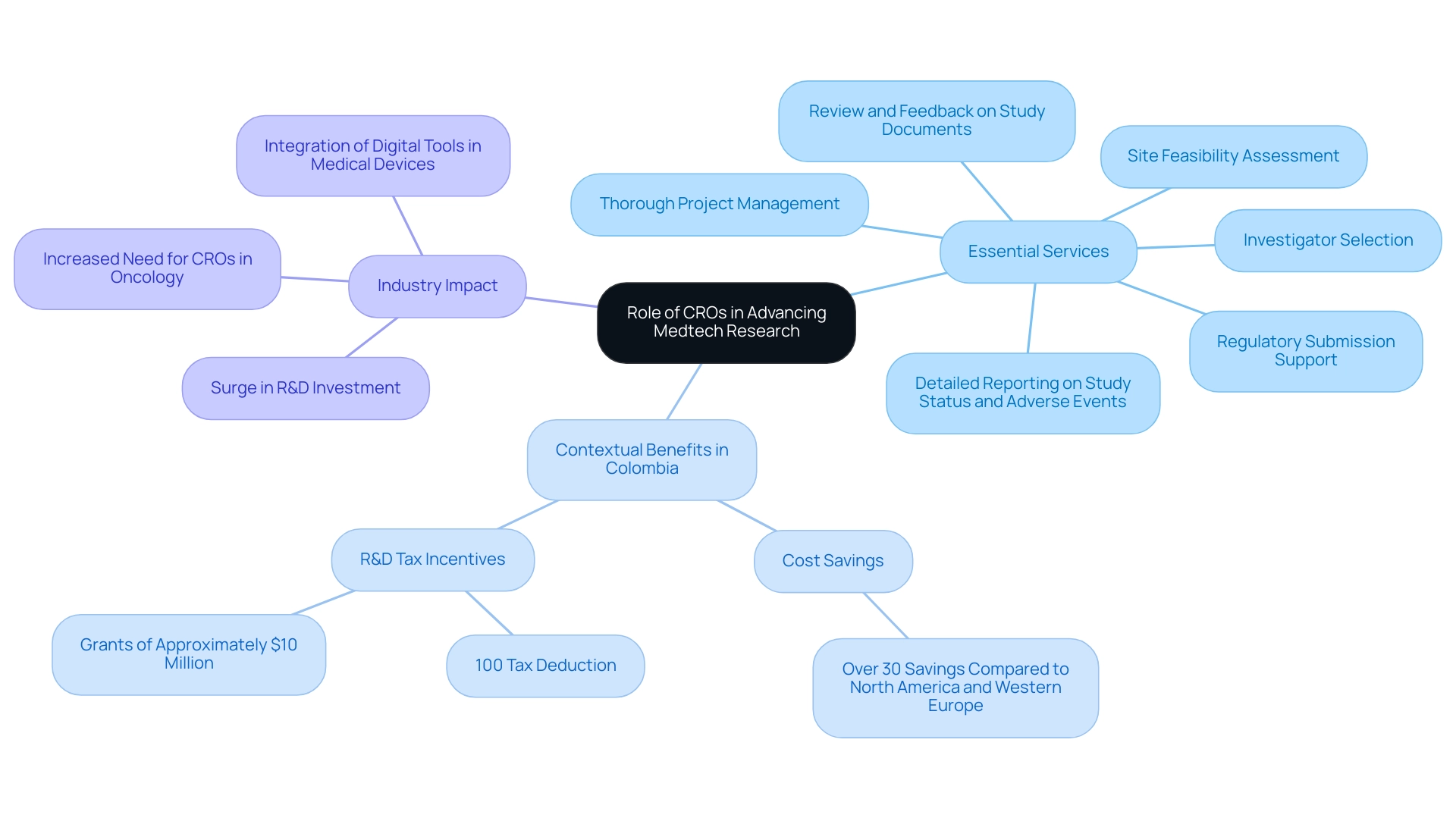
Contract Research Organizations (CROs) such as bioaccess® play a pivotal role in advancing Medtech research by providing a comprehensive suite of essential services, including:
- Site feasibility assessment
- Investigator selection
- Regulatory submission support
- Review and feedback on study documents
- Thorough project management
- Detailed reporting on study status and adverse events
This is vital for both established companies and emerging Medtech startups navigating the complexities of clinical studies. Particularly in Colombia, where the healthcare system is highly regarded and offers significant cost savings of over 30% compared to North America and Western Europe, CROs help streamline the approval process—typically taking only 90-120 days for IRB/EC and INVIMA review.
As Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, reflects on his experience with bioaccess® during its initial human evaluation, the operational efficiencies and regulatory compliance expertise offered by CROs allow researchers to focus on their scientific objectives, while also addressing the common challenges faced by medical device startups, such as regulatory hurdles and financial constraints.
Furthermore, Colombia provides R&D tax incentives, such as a 100% tax deduction and grants of approximately $10 million for investments in science, technology, and innovation projects, further boosting its attractiveness as a location for research studies. In the context of a thriving Latin American market, where quality healthcare and patient recruitment are enhanced by universal coverage, CROs are becoming increasingly vital for the successful implementation of research studies, particularly in the area of Medtech in Latin American trials and precision medicine.
Challenges in Clinical Research Management for Medtech Trials
Overseeing research in Medtech in Latin American trials entails maneuvering through a landscape characterized by particular challenges, especially in patient recruitment, timeline management, and data integrity. The recruitment of a suitable patient population remains a critical obstacle in Medtech in Latin American trials, often leading to significant delays in study timelines, particularly within specialized segments of the Medtech industry. Scott Gray, CEO of Clincierge, aptly notes,
By better understanding a patient’s needs, preferences, and participation barriers, a more efficient and patient-friendly research model can be constructed, ensuring a more positive overall experience.
This insight underscores the necessity of tailoring recruitment strategies to meet patient expectations.
Moreover, bioaccess® offers extensive research management services customized to the specific needs of the American market, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Post-Market Follow-Up Studies (PMCF)
With more than 20 years of experience in Medtech in Latin American trials, bioaccess® is well-prepared to tackle the complexities of these studies. The partnership between bioaccess™ and Caribbean Health Group aims to position Barranquilla as a premier site for Medtech in Latin American trials, with strong backing from Colombia's Minister of Health, who is actively endorsing this initiative.
This collaboration is pivotal in addressing the challenges of limited awareness and distrust among potential participants in Medtech in Latin American trials, as highlighted in a case study titled 'Challenges and Solutions in Recruitment and Retention.'
Solutions such as:
- education campaigns
- patient-friendly study designs
- financial incentives
- continuous engagement through digital platforms
have been proposed to mitigate dropout rates. Furthermore, the reliance on digital media—where companies like Dell reportedly source 80% to 90% of their advertising—has transformed how research teams engage potential participants. Data collected through digital interactions not only improves awareness campaigns but also assists in identifying appropriate candidates while adhering to privacy regulations.
To effectively manage multiple projects, research directors must cultivate strong organizational skills and encourage clear communication among team members, emphasizing the flexibility and specialized knowledge required for successful Medtech in Latin American trials.
To tackle these challenges, employing strategic planning, utilizing digital project management tools, and fostering a collaborative team environment can greatly enhance the execution and success of medical studies, especially for Medtech in Latin American trials. A focus on timeline management is also essential, as delays in recruitment can extend the overall duration of studies, affecting both costs and outcomes.
Future Trends and Innovations in Medtech Clinical Trials
The landscape of Medtech in Latin American trials is on the verge of substantial change, especially due to technological progress and creative methodologies. Digital health solutions, such as telemedicine and remote patient monitoring, are rapidly gaining traction, facilitating more flexible and efficient study designs. For instance, nearly 200 clinical studies currently registered for psychedelics highlight the pressing need for adaptive research strategies.
Furthermore, the incorporation of artificial intelligence and big data analytics is transforming patient selection and data analysis, ultimately resulting in enhanced outcomes. However, US Medtech companies face critical challenges in Medtech in Latin American trials, including:
- Professionalism
- Language barriers
- The lack of CRO corporate structures, which hinder effective collaboration with local hospitals.
As highlighted by Arda Ural, PhD, a notable individual in the Americas Life Sciences field, the future of research studies will necessitate that scientists stay adaptable and knowledgeable.
This adaptability is crucial for leveraging emerging technologies and methodologies effectively, addressing these challenges. Partnerships, such as those between Greenlight Guru and bioaccess™, are crucial in closing these gaps, ensuring thorough management services that encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Project oversight.
bioaccess® specializes in:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Post-Market Clinical Follow-Up Studies (PMCF)
among others, which directly address the unique challenges faced in Medtech in Latin American trials.
This approach not only enhances operational efficiency and participant recruitment but also aligns with the need for Medtech companies to explore direct-to-consumer offerings as a new revenue stream opportunity, ultimately driving better financial performance in their clinical trials.
Conclusion
Latin America's Medtech sector is rapidly evolving, presenting a unique blend of opportunities and challenges for stakeholders. The region's emergence as a hub for innovation in medical devices and digital health technologies is underscored by the growing number of startups and clinical trials, particularly in countries like Colombia, Brazil, and Mexico. Strategic partnerships are essential for navigating the regulatory complexities and resource limitations that companies encounter, as evidenced by initiatives aimed at establishing Barranquilla as a key destination for clinical research.
The advantages of conducting clinical trials in Latin America are compelling:
- Cost-effectiveness
- Diverse patient populations
- Robust healthcare infrastructure
These factors contribute to the region's attractiveness for clinical research. With projected growth in the clinical trials market and enhanced collaboration among biopharmaceutical companies, the potential for successful trial execution is significant. Moreover, the role of Contract Research Organizations (CROs) is pivotal in facilitating efficient study management and regulatory compliance, further strengthening the region's position in the global Medtech landscape.
As the industry moves forward, embracing innovations such as telemedicine and artificial intelligence will be crucial in overcoming existing hurdles. The adaptability of researchers and the strategic use of emerging technologies will define the future of clinical trials in Latin America. By understanding the intricacies of this evolving market and fostering collaboration, stakeholders can unlock the full potential of the Medtech sector, driving advancements that will ultimately benefit healthcare systems and patient outcomes across the region.




