Overview
The article focuses on the opportunities in US-Latin American MedTech collaboration, emphasizing how these partnerships can leverage diverse resources and expertise to address regional healthcare challenges. It supports this by detailing successful collaborations, such as those between US companies and Latin American startups, which enhance innovation, accelerate regulatory processes, and ultimately improve patient outcomes in the region.
Introduction
The collaboration between the United States and Latin America in the MedTech sector represents a pivotal opportunity to harness diverse resources and expertise, ultimately enhancing healthcare delivery across the region. As partnerships between innovative companies and local institutions flourish, they pave the way for groundbreaking medical devices tailored to address the unique health challenges faced by Latin American populations.
From joint ventures that merge cutting-edge technology with cultural insights to strategic initiatives aimed at overcoming regulatory hurdles, the landscape is ripe for transformation. This article delves into the importance of these collaborations, the key initiatives driving them, and the challenges that must be navigated to fully realize their potential, offering a comprehensive overview of the future trends and opportunities that lie ahead in US-Latin American MedTech collaboration.
The Importance of US-Latin American MedTech Collaboration
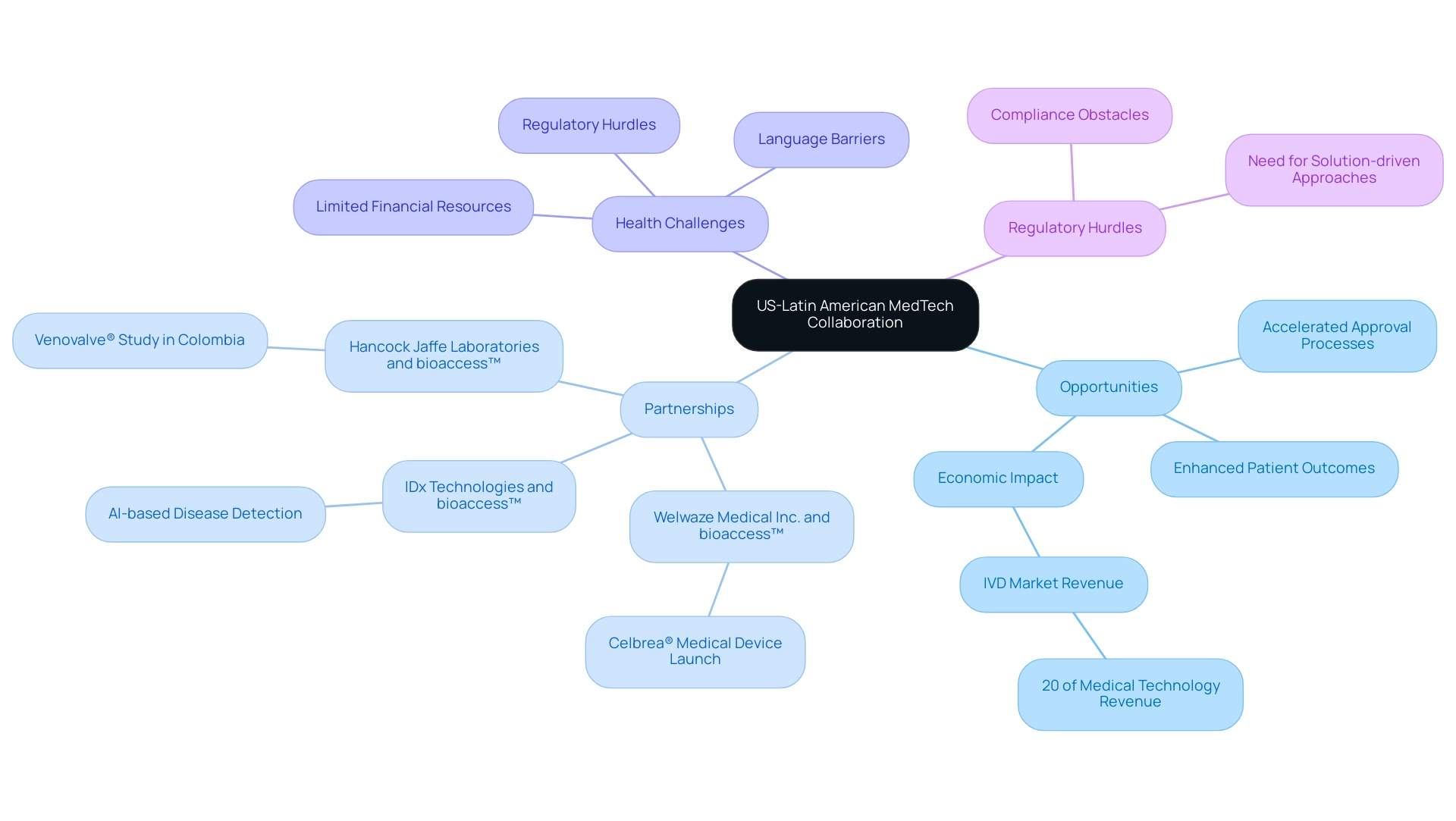
The collaboration between the US and South America in the MedTech sector creates opportunities in US-Latin American MedTech collaboration, essential for maximizing diverse resources and expertise while enhancing market access. Such partnerships—like those between Welwaze Medical Inc. and bioaccess™ for the Celbrea® medical device launch, and IDx Technologies with bioaccess™ to identify Latin American ophthalmology centers for AI-based disease detection—illustrate the opportunities in US-Latin American medtech collaboration by merging advanced technologies from the US with the distinctive healthcare needs and insights prevalent in Latin American countries. For example, joint ventures can yield the creation of medical devices specifically designed to tackle widespread health challenges, ultimately enhancing patient outcomes.
Furthermore, the opportunities in US-Latin American medtech collaboration are instrumental in accelerating the testing and regulatory approval processes for new technologies, as exemplified by Hancock Jaffe Laboratories selecting bioaccess™ for its first-in-human Venovalve® study in Colombia. This synergy not only facilitates faster integration of innovations into healthcare systems but also significantly improves the overall delivery of healthcare across the region, showcasing the opportunities in US-Latin American medtech collaboration. As emphasized by Jennifer Mendoza, a research specialist in health and medical technology, in-vitro diagnostics (IVD) alone made up 20 percent of the medical technology market revenue last year, highlighting the opportunities in US-Latin American medtech collaboration and its potential economic effect.
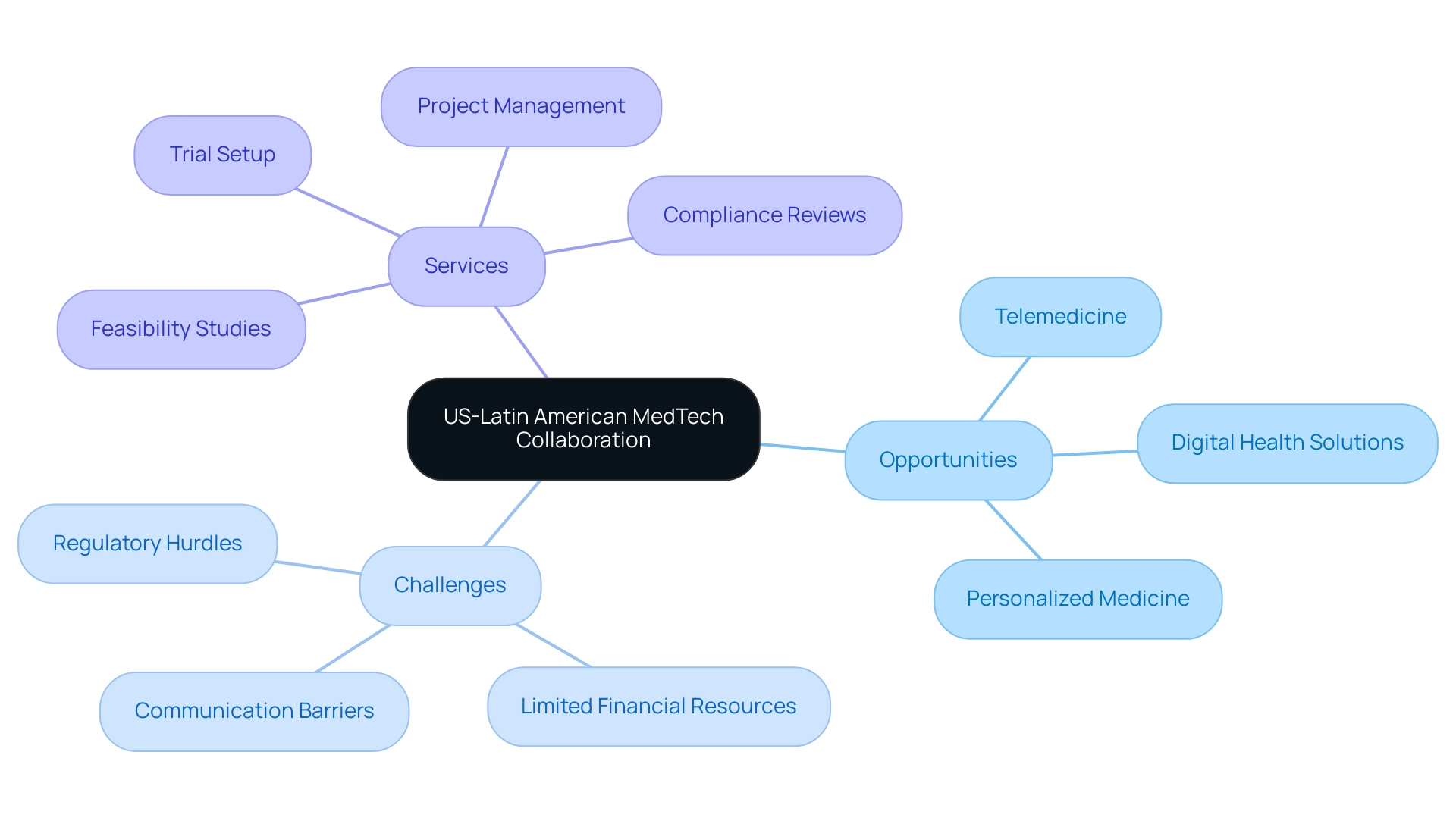
According to Global Health Intelligence, the number of ultrasound devices in hospitals throughout chosen South American nations is expected to rise in 2024, further demonstrating the expanding medical technology landscape. However, healthcare technology startups in Latin America face challenges such as compliance obstacles, limited financial resources, and language barriers, which create opportunities in US-Latin American medtech collaboration to enhance research and innovation. The developing healthcare technology partnership landscape highlights the significance of telemedicine and digital wellness solutions, which offer potential for innovation and enhanced patient results, despite current compliance challenges.
It is crucial to address these regulatory hurdles to fully leverage the potential of medical technology innovations in the region.
Key Initiatives Driving MedTech Collaboration
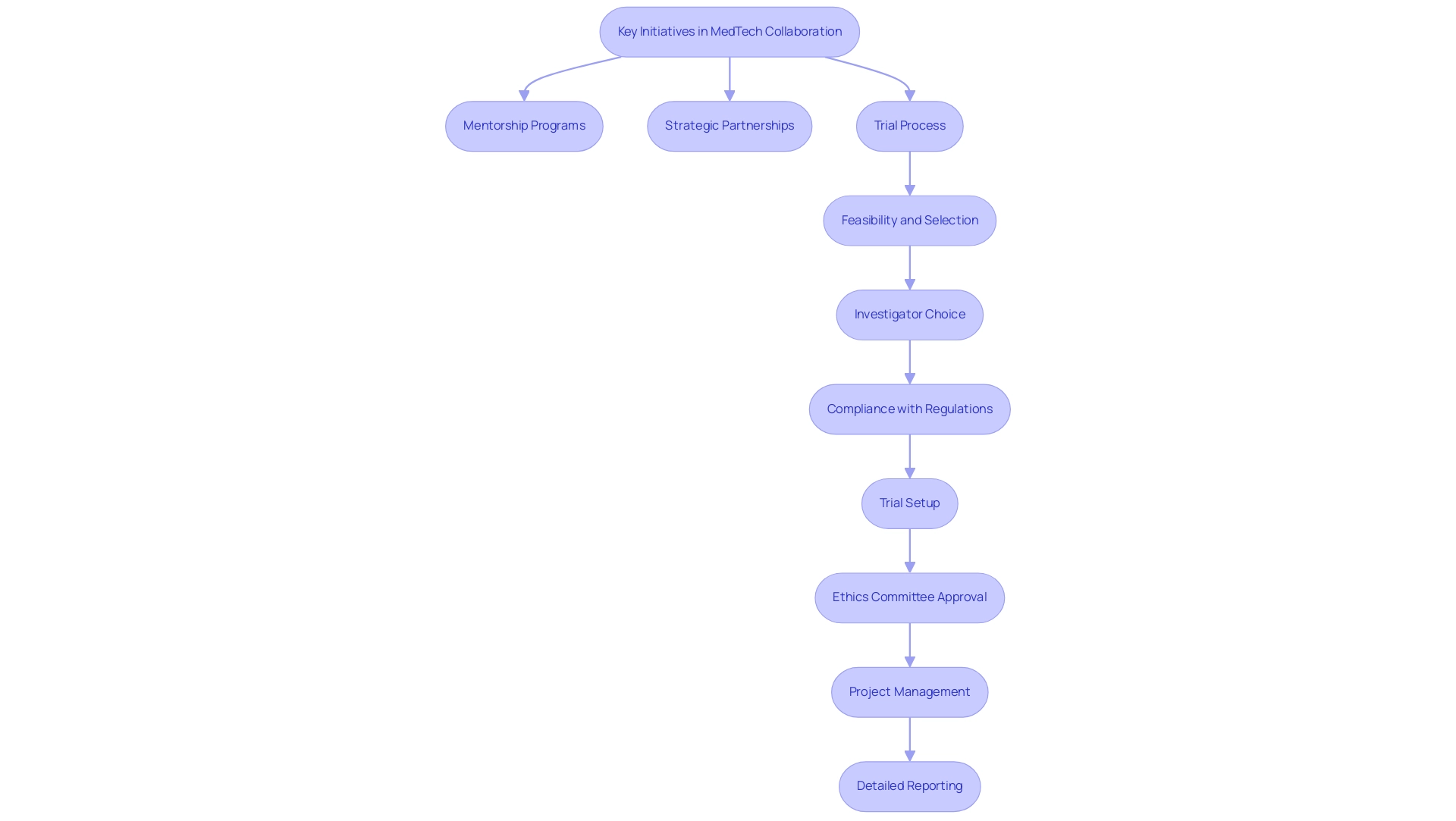
A range of initiatives is currently creating opportunities in US-Latin American medtech collaboration. Among these, mentorship programs are crucial as they forge connections between emerging MedTech companies and seasoned industry leaders, thereby facilitating vital knowledge transfer and skill development. Mariana Romero Roy, Senior Director of Business Intelligence for GHI, emphasizes the importance of these connections, stating,
This work has honed her expertise in market trends and helping sales teams uncover opportunities in the region.
Furthermore, strategic partnerships between universities and research institutions are pivotal, enabling collaborative research efforts aimed at tackling specific healthcare challenges prevalent in diverse populations. Significantly, the extensive procedure of progressing medical device trials entails:
- Feasibility and selection of research sites
- Investigator choice
- Compliance with regulations
- Trial setup
- Ethics committee approval
- Project management
- Detailed reporting on study results, including study status and adverse events
This is especially clear in Colombia, where the competitive advantages for first-in-human clinical trials include cost efficiency, swift approval processes, high-quality healthcare, effective patient recruitment, and appealing R&D tax incentives.
Additionally, the impact of Medtech clinical studies on local economies is significant, contributing to job creation, economic growth, and improved healthcare systems while fostering international collaboration. Navigating compliance environments and cultural differences is essential for maximizing the potential of these collaborations, ensuring that diverse perspectives are effectively integrated into innovation processes. Industry conferences and trade shows serve as essential platforms for stakeholders to network, exchange insights, and explore opportunities in US-Latin American medtech collaboration, which ultimately propels innovation within the medical technology sector.
Collectively, these initiatives not only address immediate healthcare needs but also lay the groundwork for sustainable growth and advancement in the industry.
Navigating the Regulatory Landscape for MedTech in Latin America
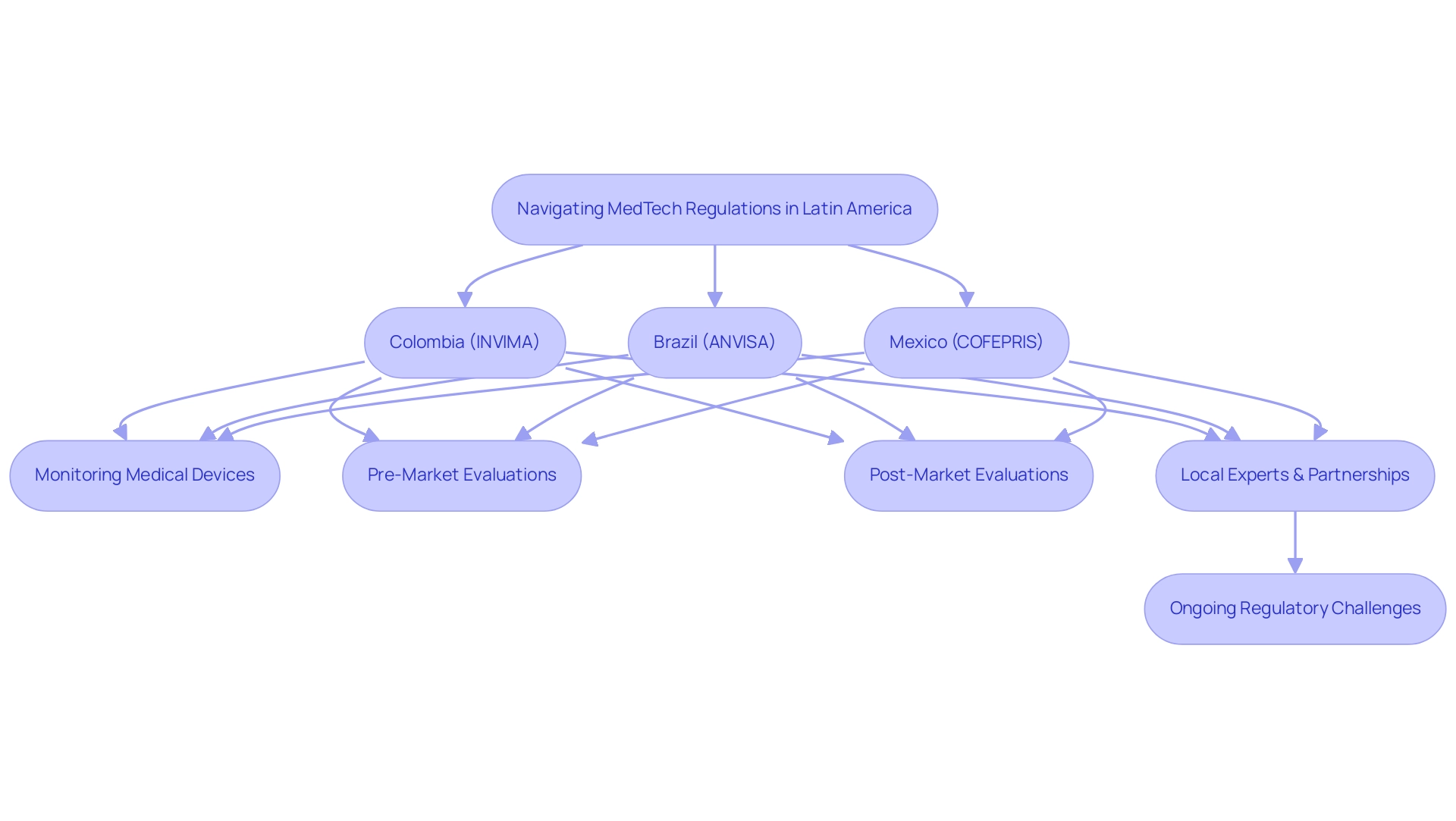
Navigating the compliance landscape for MedTech in Latin America requires a nuanced understanding of country-specific requirements alongside regional harmonization efforts. Each nation upholds its own governing entity, with Colombia's INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos) being a key player established in 1992 under the Ministry of Health and Social Protection. INVIMA oversees the marketing and manufacturing of health products, ensuring compliance with safety and efficacy standards, and has been classified as a Level 4 health authority by PAHO/WHO, indicating its competence in health regulation.
A crucial component of INVIMA's functions is its Directorate for Medical Devices and other Technologies, which is responsible for:
- Monitoring medical devices
- Suggesting technical standards
- Managing pre- and post-market evaluations to ensure product safety and effectiveness
This governing framework exemplifies the variations in approval processes across the region, as seen with Brazil's ANVISA and Mexico's COFEPRIS, each with unique compliance protocols. The latest Pulse of the Healthcare Technology Industry report highlights the sector's rapid advancements, particularly with breakthroughs in AI promising enhanced device functionality and personalization.
To stay compliant, stakeholders should actively monitor these regulations and consider partnering with local experts such as Katherine Ruiz, an authority in compliance affairs for medical devices and in vitro diagnostics in Colombia, or firms like bioaccess®, which lead in Medtech clinical research with a focus on innovation and compliance excellence. Furthermore, emerging initiatives aimed at harmonizing regulations across South American countries could create opportunities in US-Latin American MedTech collaboration by streamlining the approval process for MedTech devices and fostering cross-border collaboration. Significantly, the orthopedic devices market in South America, valued at 3.6 billion USD, highlights the potential for growth and innovation in this governance context, especially as companies navigate INVIMA's comprehensive oversight.
Furthermore, a recent case study named 'Distribution of Healthtech Startups in Latin America (2023)' highlights the varied landscape of Healthtech startups, stressing the necessity for customized oversight strategies in this evolving market. This case study demonstrates how innovative startups are adjusting to compliance requirements, which can significantly affect their market entry and growth potential. However, stakeholders must remain vigilant regarding ongoing regulatory challenges, which can significantly impact the speed and efficiency of introducing innovative medical technology solutions to market.
Overcoming Cultural and Operational Challenges in MedTech Partnerships
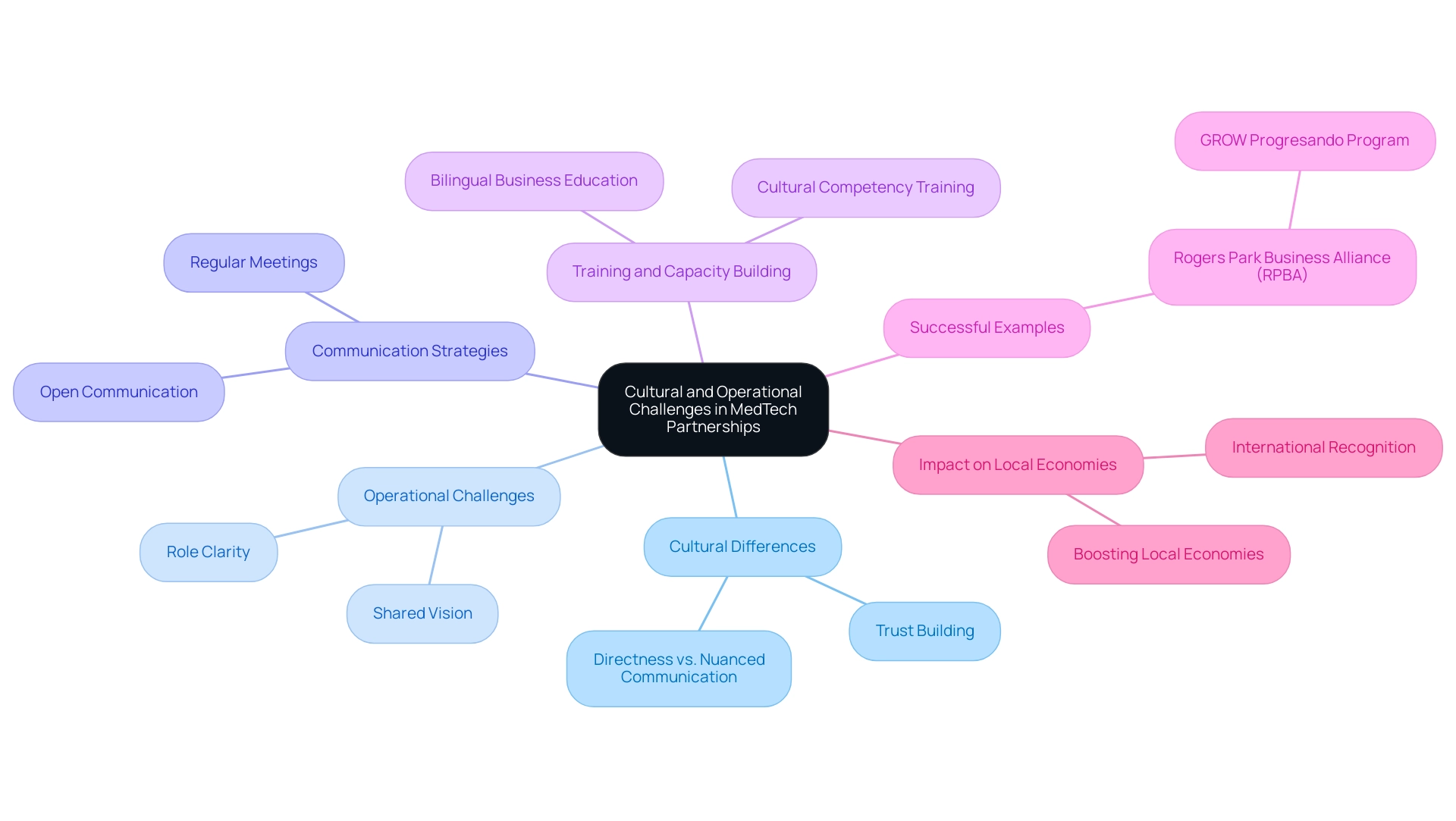
Cultural differences and operational challenges present significant hurdles to realizing opportunities in US-Latin American MedTech collaboration. Notably, communication styles can differ markedly, often leading to misunderstandings. For instance, while some cultures may prioritize directness in communication, others may rely on a more nuanced approach, which can affect decision-making processes and expectations regarding timelines.
Recent statistics highlight that 13% of Latino-owned small businesses face failure within their first year, compared to 10% for their White counterparts, illustrating the unique pressures within these partnerships. Julio Martinez-Clark, CEO of bioaccess and a strong supporter of Medtech clinical research in the region, emphasizes the need for operationalizing clinical trials and mentoring startups to harness the opportunities in US-Latin American Medtech collaboration. As the owner of a software company catering to the legal sector remarked, "There’s a network effect phenomenon in Miami that comes with being surrounded by clients and colleagues from South America," emphasizing the significance of local connections.
To effectively navigate these challenges, fostering open communication and building trust among partners is essential. Regular meetings and collaborative decision-making can serve as effective tools to mitigate potential misunderstandings. Implementing cultural competency training for teams will enhance mutual respect and understanding, thereby facilitating smoother collaboration.
Furthermore, establishing clear roles and responsibilities, along with a shared vision for the partnership, can significantly enhance operational efficiency. A practical example is the Rogers Park Business Alliance (RPBA) in Chicago, which fosters local partnerships and provides bilingual business education to Latino entrepreneurs through its GROW Progresando program. This initiative enhances the skills and networks of Latino entrepreneurs, facilitating connections with local financial institutions and legal resources.
Additionally, successful Medtech clinical studies can lead to international recognition, further boosting local economies and fostering global collaboration. This comprehensive strategy not only tackles cultural obstacles but also establishes a strong basis for successful healthcare partnerships across borders, creating opportunities in US-Latin American Medtech collaboration as advancements in health services continue to change lives in the southern continent. Monica Mora, Chief Operating Officer focusing on operations, logistics, and compliance strategies for medical device firms in Latin America, plays a vital role in navigating these complexities, ensuring that partnerships are both effective and in accordance with local regulations.
Future Trends and Opportunities in US-Latin American MedTech Collaboration
The landscape of [[US-Latin American MedTech collaboration](https://bioaccessla.com/home) presents numerous opportunities](https://blog.bioaccessla.com/why-exploring-med-tech-collaboration-between-the-us-and-latin-america-is-essential-for-innovation) in US-Latin American MedTech collaboration, driven by rapid technological advancements and changing healthcare needs. Challenges such as regulatory hurdles, limited financial resources, and communication barriers have historically impeded progress. However, innovative solutions and collaborations, particularly between organizations like Greenlight Guru and bioaccess™, are paving the way for accelerated clinical trials and enhanced medical device studies.
Bioaccess offers critical services, including:
- Feasibility studies
- Trial setup
- Project management
- Compliance reviews
These services are essential for navigating the complexities of clinical research in the region. Innovations in telemedicine, digital health solutions, and personalized medicine are taking center stage, creating a plethora of opportunities for collective expertise in these domains. The COVID-19 pandemic has served as a catalyst for this transformation; with over 22 million cases reported in the region, including more than 5.3 million confirmed cases in Argentina alone, the demand for advanced medical technologies surged, leading to improved patient monitoring and data management.
Additionally, with dropout rates in Central and South America being one-third of those in the U.S. and EU, the region showcases significant potential for growth and innovation. While concerns regarding data security and language barriers remain a barrier, the increasing focus on patient-centered care underscores the necessity for innovative MedTech solutions tailored to address specific regional health challenges. Stakeholders must remain agile and proactive in exploring these emerging trends to fully capitalize on the vast opportunities in US-Latin American MedTech collaboration for 2024 and beyond.
As Latin American medical research and development reshapes the healthcare landscape, sustained innovation is essential to unlock new markets and meet evolving patient needs.
Conclusion
The collaboration between the United States and Latin America in the MedTech sector presents a transformative opportunity to enhance healthcare delivery and innovation across the region. By leveraging diverse resources and expertise, partnerships between innovative companies and local institutions have led to the development of medical devices tailored to address specific health challenges. Key initiatives, such as mentorship programs and strategic university partnerships, are instrumental in fostering knowledge transfer and facilitating research that directly impacts patient outcomes.
Navigating the complex regulatory landscape remains a critical component of these collaborations. Understanding country-specific requirements and engaging with local experts can streamline the approval process, allowing for quicker integration of advanced technologies into healthcare systems. Moreover, addressing cultural differences and operational challenges through open communication and trust-building efforts is essential for successful partnerships.
Looking ahead, the MedTech landscape in Latin America is poised for significant growth, driven by advancements in telemedicine and personalized medicine. Stakeholders are encouraged to embrace innovative solutions that can overcome existing barriers and capitalize on emerging trends. As the region continues to evolve, sustained collaboration will be vital in unlocking new markets and improving healthcare for diverse populations. The future of US-Latin American MedTech collaboration is bright, promising enhanced patient care and economic development through strategic partnerships and innovative approaches.
Frequently Asked Questions
What are the main benefits of US-Latin American MedTech collaboration?
US-Latin American MedTech collaboration creates opportunities for maximizing diverse resources and expertise, enhancing market access, accelerating testing and regulatory approval processes, and improving overall healthcare delivery.
Can you provide examples of successful collaborations in the MedTech sector?
Examples include Welwaze Medical Inc. partnering with bioaccess™ for the Celbrea® medical device launch, and IDx Technologies collaborating with bioaccess™ to identify Latin American ophthalmology centers for AI-based disease detection.
How does collaboration impact the development of medical devices?
Joint ventures can lead to the creation of medical devices tailored to address widespread health challenges, ultimately enhancing patient outcomes.
What role does bioaccess™ play in MedTech collaborations?
Bioaccess™ has been selected by Hancock Jaffe Laboratories for its first-in-human Venovalve® study in Colombia, facilitating faster integration of innovations into healthcare systems.
What challenges do healthcare technology startups in Latin America face?
Startups encounter compliance obstacles, limited financial resources, and language barriers, which can hinder research and innovation.
How important are mentorship programs in the MedTech sector?
Mentorship programs are crucial as they connect emerging MedTech companies with experienced industry leaders, facilitating knowledge transfer and skill development.
What are the steps involved in progressing medical device trials?
The steps include feasibility and selection of research sites, investigator choice, compliance with regulations, trial setup, ethics committee approval, project management, and detailed reporting on study results.
What advantages does Colombia offer for first-in-human clinical trials?
Colombia offers cost efficiency, swift approval processes, high-quality healthcare, effective patient recruitment, and attractive R&D tax incentives.
How do MedTech clinical studies impact local economies?
They contribute to job creation, economic growth, and improved healthcare systems while fostering international collaboration.
What is the significance of industry conferences and trade shows in MedTech collaboration?
They serve as essential platforms for stakeholders to network, exchange insights, and explore opportunities, ultimately propelling innovation within the medical technology sector.

