Introduction
In the realm of medical device regulation, Post-Market Clinical Follow-Up (PMCF) stands as a critical component for ensuring the ongoing safety and performance of products once they reach the market.
In Colombia, the significance of PMCF is underscored by stringent regulatory requirements that aim to gather real-world evidence, thereby enhancing the credibility of clinical research and safeguarding patient welfare.
As Medtech companies navigate the complexities of compliance, including regulatory hurdles and language barriers, the role of effective PMCF methodologies becomes increasingly paramount.
This article delves into the multifaceted aspects of PMCF in Colombia, exploring the design, implementation, and analysis of surveys, as well as the importance of robust data management practices.
Through insights from industry experts and case studies, the discussion highlights how collaboration with experienced clinical research organizations can facilitate streamlined processes and foster innovation in the Latin American Medtech landscape.
Introduction to Post-Market Clinical Follow-Up (PMCF) in Colombia
Post-Market Clinical Follow-Up denotes the systematic gathering of information concerning the performance and safety of medical devices after they have been introduced to the market. In Colombia, adherence to regulatory requirements makes Post-Market Clinical Follow-Up Colombia essential for collecting real-world evidence that supports the ongoing evaluation of a medical product's benefits and risks. This process not only ensures patient safety but also aligns with global practices, enhancing the credibility of research in the region.
Medtech firms in Latin America encounter particular obstacles, including regulatory challenges and language barriers, which can complicate the Post-Market Clinical Follow-Up Colombia process. With the support of bioaccess®, a prominent US-based CRO recognized for its economical services and capacity to hasten study outcomes, post-market follow-up includes various methodologies, such as:
- Surveys
- Registries
- Studies
These methodologies offer invaluable insights into product performance in diverse populations and settings. The collaboration between Greenlight Guru and bioaccess™ further accelerates Medtech innovations, as evidenced by PAVmed's successful First In-Human Study in Colombia, showcasing the potential of streamlined clinical trials in Latin America.
Implementing PMCF Surveys: Timing and Rationale
The timing of Post-Market Clinical Follow-Up Colombia surveys is influenced by several factors, including the medical device's characteristics, intended use, and the regulatory frameworks established by Colombian authorities such as INVIMA, which serves as a Level 4 health authority recognized by PAHO/WHO. Typically, post-market clinical follow-up surveys should be initiated after a product's initial market introduction, occurring at predetermined intervals or following significant modifications to the device or its indications. This proactive approach is essential for collecting ongoing safety and performance data, which assists manufacturers in making informed decisions and facilitates timely updates to risk assessments.
Notably, industry expert Josie Wyss from Cochlear Ltd. emphasizes the importance of these evaluations:
we report the challenges and successes in design and implementation and make recommendations for future registries.
This perspective emphasizes the critical nature of post-market clinical follow-up surveys in ensuring patient safety and product reliability. A recent example is the IROS registry, which successfully recruited over 1500 hearing implant users, demonstrating how post-market clinical follow-up surveys can provide valuable insights into patient-related outcomes in the hearing sector.
Additionally, these surveys may be initiated due to specific safety concerns or adverse events, reaffirming the commitment to prioritizing patient safety throughout the device lifecycle. Moreover, following the methodological guidelines for patient registry governance issued by the National Institute of Public Health is crucial, highlighting that efficient data access for contributors, as demonstrated in the case 'Data Access for Contributors,' greatly improves the usability of data for local reporting requirements. Lastly, ensuring that all evaluations related to Post-Market Clinical Follow-Up Colombia comply with Colombian regulatory requirements is crucial for maintaining alignment with local standards and best practices.
At bioaccess®, we provide extensive trial management services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
to ensure the successful execution of Post-Market Clinical Follow-Up Colombia in Latin America.
Designing Effective PMCF Surveys
Creating effective Post-Market Clinical Follow-Up Colombia surveys necessitates careful attention to the target population, specific objectives, and selected collection methods. Establishing clear research questions that align with both regulatory requirements and clinical needs is paramount. Surveys should encompass both quantitative and qualitative information, enabling a comprehensive understanding of device performance and user experience.
Utilizing validated questionnaires enhances the credibility of the information collected, while ensuring that the language used is accessible to all respondents promotes engagement and clarity. Moreover, selecting the appropriate mode of collection—whether online, via telephone, or in-person—can significantly impact response rates and overall quality. Recent case analyses showcased in a webinar emphasize the significance of Post-Market Clinical Follow-Up Colombia for both high-risk and lower-risk devices, illustrating its function in continuous information gathering and risk confirmation.
Significantly, innovative methods, such as retrospective post-market clinical follow-up surveys, have demonstrated effectiveness in collecting real-world data, further aiding the continuous validation of medical devices in the market. As highlighted by specialists from bioaccess®, who possess more than 20 years of Medtech expertise, their extensive management services for trials—including feasibility assessments, site selection, trial setup, and regulatory compliance with INVIMA—ensure that all procedures align with Post-Market Clinical Follow-Up Colombia best practices. This collaboration emphasizes the importance of effective Post-Market Clinical Follow-Up Colombia survey design in maintaining compliance and enhancing product offerings, with methodologies tailored to meet specific regulatory and clinical needs.
Data Collection and Management in PMCF
Effective information gathering and administration are essential to the success of evaluations in Post-Market Clinical Follow-Up Colombia. A well-defined framework for information gathering is essential, including clearly delineating roles and responsibilities among the research team to ensure accountability and efficiency. With over 20 years of expertise in Medtech, our services at bioaccess® include:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Project management
All tailored to enhance the execution of PMCF initiatives.
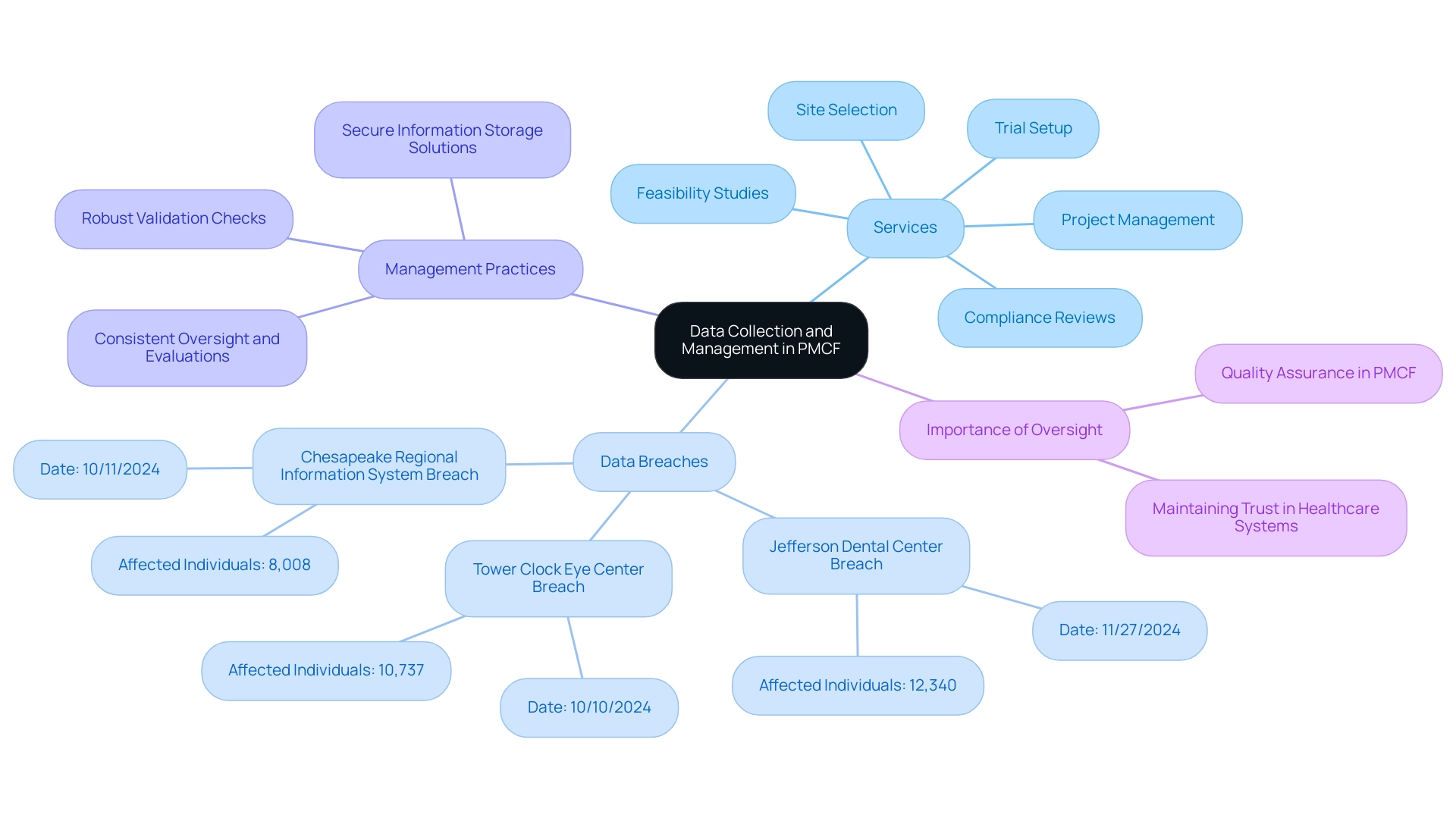
Recent statistics highlight the urgency of secure information management practices; for instance, Jefferson Dental Center, Inc. reported a breach affecting 12,340 individuals, while Tower Clock Eye Center experienced an incident impacting 10,737 individuals. These cases emphasize the critical need for robust information protection strategies. Leveraging secure information storage solutions not only protects patient confidentiality but also guarantees compliance with INVIMA's regulations and the recently updated protection laws in Colombia, which are crucial for clinical studies.
As AHA President and CEO Rick Pollack emphasized in a recent podcast, effective information management is vital for maintaining trust in healthcare systems. Implementing robust validation checks is another critical practice, as it aids in identifying discrepancies or errors during entry, thereby enhancing the overall integrity of the collected information. Consistent oversight and evaluations of the information management procedure are essential for upholding quality assurance, which directly aids the successful implementation of Post-Market Clinical Follow-Up Colombia.
Furthermore, bioaccess® oversees a variety of research, such as:
- Early-Feasibility Assessments
- First-In-Human Trials
- Pilot Trials
Ensuring thorough supervision throughout the trial process. As the terrain of information management in clinical research changes, these strategies become more vital for ensuring both safety and effectiveness in PMCF analysis, especially considering the ramifications highlighted by the Tower Clock Eye Center breach.
Analyzing PMCF Data for Insights
Once information collection is complete, a thorough analysis must be conducted to identify trends, correlations, and potential safety signals. Utilizing appropriate statistical methods ensures that the analysis aligns with the study objectives. Qualitative information should also be examined to gain deeper insights into patient experiences and perceptions, further enhancing the robustness of the findings.
Collaborating with biostatisticians or data analysts, along with the extensive expertise of bioaccess® in managing trials—including Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies—can offer essential insights. Our service capabilities include:
- Feasibility and selection of research sites
- Compliance reviews
- Trial setup
- Project management
This ensures a comprehensive approach to trial management. The results of the analysis should be documented comprehensively, highlighting significant findings that may necessitate further investigation or lead to modifications in product labeling, usage guidelines, or additional studies.
Katherine Ruiz, our regulatory affairs expert, brings invaluable experience from her tenure at INVIMA, where she facilitated market clearances for medical innovations, further strengthening our commitment to compliance and excellence in clinical research.
Reporting PMCF Findings to Regulatory Authorities
Following the analysis of Post-Market Clinical Follow-Up Colombia data, it is crucial to report the findings to relevant regulatory authorities, particularly INVIMA (Colombia National Food and Drug Surveillance Institute). As a Level 4 health authority recognized by PAHO/WHO, INVIMA plays a vital role in ensuring the safety and efficacy of medical devices. The report must encompass a comprehensive summary of the research design, methodology, findings, and any recommendations derived from the results.
This encompasses specific details regarding the comprehensive clinical trial management services, such as feasibility assessments and site selection, which are crucial for ensuring that all elements of the clinical trial process are addressed. As Dr. Daniel Shepshelovich, MD, highlights, supervision in this process is vital to ensure thoroughness and accuracy. Adhering to specific reporting timelines, as mandated by regulatory guidelines, is essential for ensuring compliance.
Notably, the number of safety communications published each year has remained stable over time, reflecting the ongoing commitment to regulatory compliance in the medical device sector. Additionally, engaging with experts like Katherine Ruiz, a Regulatory Affairs specialist with extensive experience in navigating INVIMA's requirements, can provide invaluable insights into the approval process and compliance strategies. Furthermore, referencing the case study on the public availability of ASR data underscores the importance of transparency and compliance in reporting findings related to Post-Market Clinical Follow-Up Colombia.
It is advisable to be prepared to provide additional information or clarification as needed. Engaging in open communication with regulatory bodies not only fosters a collaborative relationship but also supports the continual evaluation of product safety and efficacy, reinforcing the commitment to patient welfare and regulatory standards.
Conclusion
The Post-Market Clinical Follow-Up (PMCF) process is essential for ensuring the safety and performance of medical devices in Colombia, where adherence to regulatory requirements is paramount. The systematic collection of real-world evidence through PMCF not only enhances the credibility of clinical research but also plays a crucial role in safeguarding patient welfare. As Medtech companies face challenges such as regulatory hurdles and language barriers, effective methodologies and collaboration with experienced clinical research organizations like bioaccess® become indispensable for navigating these complexities.
The article elucidates the importance of timely and well-designed PMCF surveys, emphasizing their role in ongoing safety assessments and risk management. By employing robust data collection and management practices, companies can ensure compliance with Colombian regulations and maintain high standards of patient confidentiality. Furthermore, the analysis of PMCF data yields valuable insights that can drive product improvements and inform regulatory reporting.
In conclusion, the successful implementation of PMCF in Colombia is a multifaceted endeavor that requires careful planning, execution, and ongoing evaluation. By prioritizing effective PMCF strategies, Medtech companies can not only enhance patient safety but also contribute to the advancement of the medical device sector in Latin America. As the landscape continues to evolve, the commitment to rigorous PMCF practices will be instrumental in fostering innovation and maintaining regulatory compliance, ultimately benefiting patients and healthcare systems alike.
Frequently Asked Questions
What is Post-Market Clinical Follow-Up (PMCF) in Colombia?
PMCF refers to the systematic gathering of information regarding the performance and safety of medical devices after they have been introduced to the market. It is essential for collecting real-world evidence that supports the ongoing evaluation of a medical product's benefits and risks.
Why is PMCF important in Colombia?
PMCF is crucial for ensuring patient safety and aligning with global practices, which enhances the credibility of research in the region. It also helps in collecting data that informs regulatory compliance and product improvements.
What challenges do Medtech firms face in conducting PMCF in Latin America?
Medtech firms encounter regulatory challenges and language barriers that can complicate the PMCF process.
What methodologies are used in PMCF?
PMCF includes methodologies such as surveys, registries, and studies, which provide insights into product performance across various populations and settings.
How does the timing of PMCF surveys get determined?
The timing is influenced by factors such as the medical device's characteristics, intended use, and regulatory frameworks established by Colombian authorities like INVIMA. Surveys are typically initiated after a product's initial market introduction or following significant modifications.
What role does bioaccess® play in PMCF?
Bioaccess® is a US-based CRO that supports PMCF efforts by providing economical services and expertise to accelerate study outcomes, including trial management services like feasibility studies and compliance reviews.
What is the significance of effective survey design in PMCF?
Effective survey design is crucial for gathering comprehensive data on device performance and user experience. It involves establishing clear research questions, utilizing validated questionnaires, and selecting appropriate data collection methods.
How should data from PMCF be analyzed?
Data analysis should identify trends, correlations, and potential safety signals using appropriate statistical methods. Qualitative data should also be examined to gain deeper insights into patient experiences.
What steps are involved in reporting PMCF findings?
After analysis, findings must be reported to relevant regulatory authorities, such as INVIMA, including a summary of research design, methodology, findings, and recommendations. Compliance with reporting timelines is essential.
Why is communication with regulatory bodies important in PMCF?
Engaging in open communication with regulatory bodies fosters collaboration and supports the continual evaluation of product safety and efficacy, reinforcing commitment to patient welfare and regulatory standards.




