Introduction
The field of regulatory affairs in clinical trials is pivotal in safeguarding the welfare of participants and ensuring the integrity of research data. As the landscape of clinical research evolves, understanding the frameworks established by regulatory bodies becomes increasingly essential. This article delves into the foundations of regulatory affairs, highlighting the critical roles played by organizations such as:
- INVIMA in Colombia
- FDA in the United States
- EMA in Europe
It also addresses the complexities and challenges faced by researchers navigating this intricate landscape, while exploring the diverse career opportunities available within the field. By examining these elements, the article aims to provide a comprehensive overview of the regulatory environment that shapes clinical trials and the implications for public health and innovation.
Foundations of Regulatory Affairs in Clinical Trials
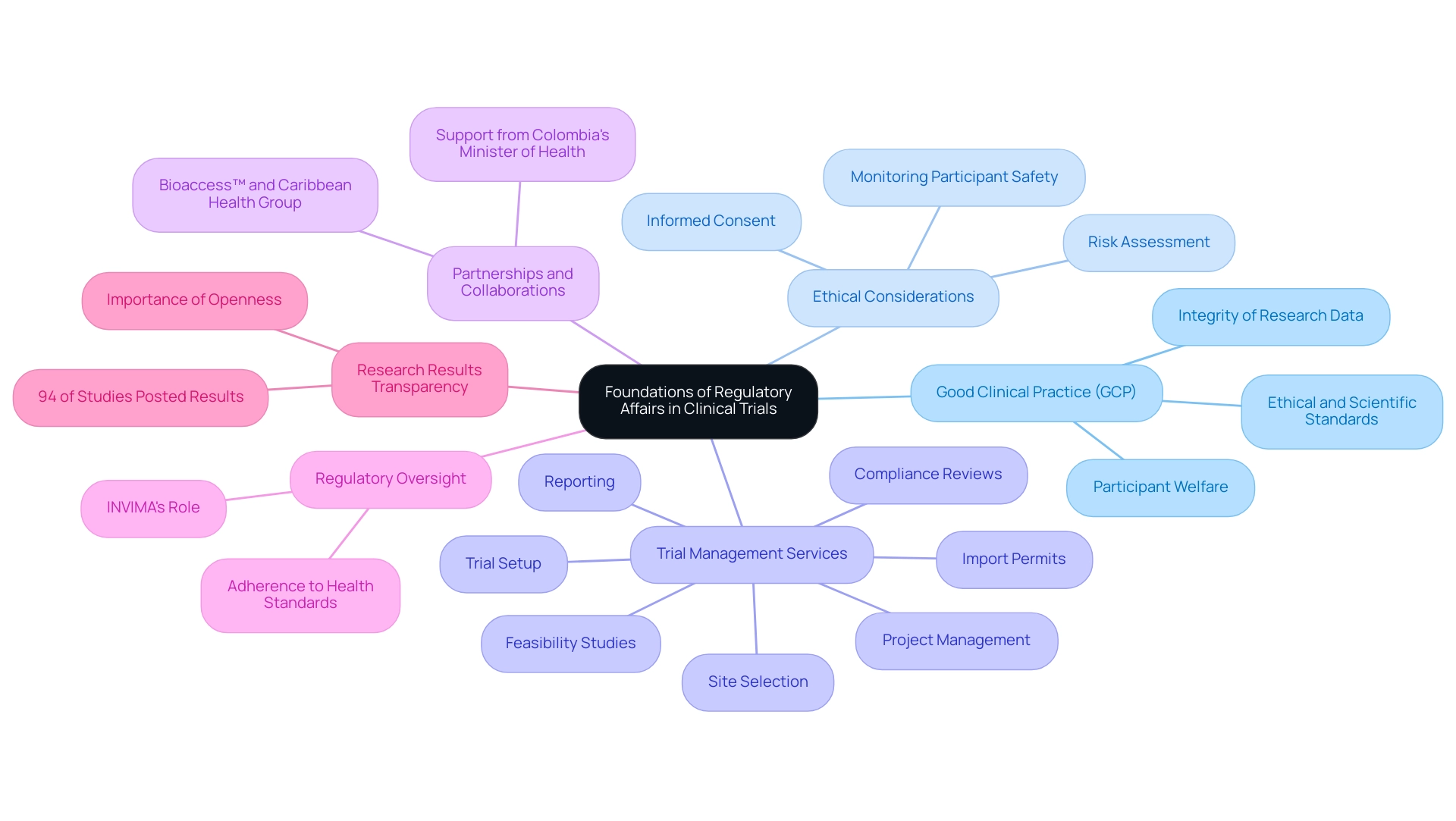
Regulatory affairs and clinical trials in research studies are essential to ensuring that investigations involving human participants comply with established policies and procedures. Central to this framework is the understanding of Good Clinical Practice (GCP), which encompasses a set of internationally recognized ethical and scientific quality standards. These standards are crucial for safeguarding participant welfare and ensuring the integrity of the research data.
In a significant development, bioaccess™ has allied with Caribbean Health Group to establish Barranquilla as a premier location for research studies in Latin America, with backing from Colombia's Minister of Health. This partnership, revealed on March 29, 2019, illustrates how cooperation can improve research environments and operational efficiencies. Additionally, as of May 2023, 94% of interventional research initiatives have shared their results, emphasizing the significance of adherence to regulations and openness in medical experiments.
Ethical considerations, particularly the necessity of informed consent, play a vital role in this process, empowering participants with the knowledge required to make informed decisions about their involvement. Moreover, comprehensive trial management services—including:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
are essential for ensuring that trials are not only scientifically sound but also ethically conducted. This is crucial for the successful approval of new therapeutics and medical devices.
Katherine Ruiz, an expert in regulatory affairs and clinical trials for Medical Devices and In Vitro Diagnostics in Colombia, emphasizes the importance of these frameworks in enhancing research environments and accelerating access to health innovations. Furthermore, INVIMA, as Colombia's National Food and Drug Surveillance Institute, plays a vital role in oversight, ensuring adherence to health standards. By aligning with these rigorous standards, researchers can facilitate a smoother path to market for innovations that ultimately benefit public health.
As Samruddhi Yardi observes, 'The adherence to Good Practice is not just a regulatory requirement; it is a commitment to the safety and rights of participants that ultimately shapes the future of research.
Key Regulatory Bodies and Their Impact on Clinical Trials
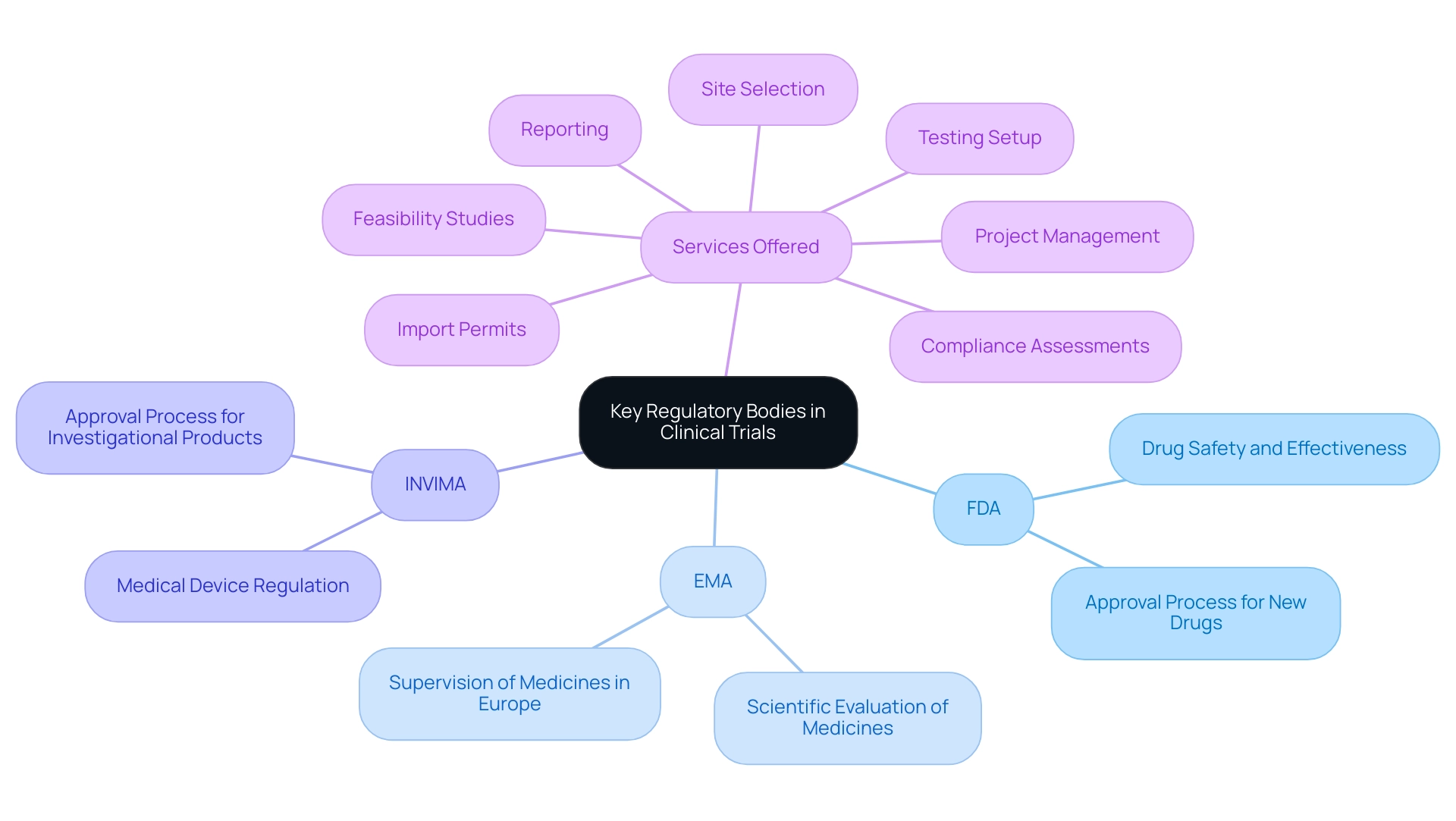
Regulatory organizations like the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe play vital roles in the supervision of research studies, including those carried out in Latin America. In Colombia, the National Food and Drug Surveillance Institute (INVIMA) serves as a Level 4 health authority recognized by PAHO/WHO, overseeing medical device regulation and the approval process for investigational products. The FDA ensures that new drugs and therapies are safe and effective prior to market entry, while the EMA is responsible for the scientific evaluation and supervision of medicines throughout Europe.
These agencies, along with INVIMA, create extensive regulations concerning regulatory affairs and clinical trials that oversee the design, execution, and reporting of research studies, protecting participant rights and ensuring data integrity. Their governing structures greatly impact the authorization procedure for experimental products within the context of regulatory affairs and clinical trials, influencing research timelines and access to novel therapies. A recent example is the approval of OGSIVEO (nirogacestat) on November 27, 2023, for the treatment of progressing desmoid tumors, demonstrating the practical impact of these regulations on timely access to new therapies.
As research specialist Katherine Ruiz highlights, comprehending these evolving frameworks is crucial for the effective management of regulatory affairs and clinical trials in Latin America. Our services include:
- Feasibility studies
- Site selection
- Compliance assessments
- Testing setup
- Import permits
- Project management
- Reporting
All aimed at promoting adherence to these legal standards.
Reach out to us now to discover how our extensive services can simplify your studies and ensure compliance with industry standards.
Navigating Regulatory Requirements for Clinical Trials
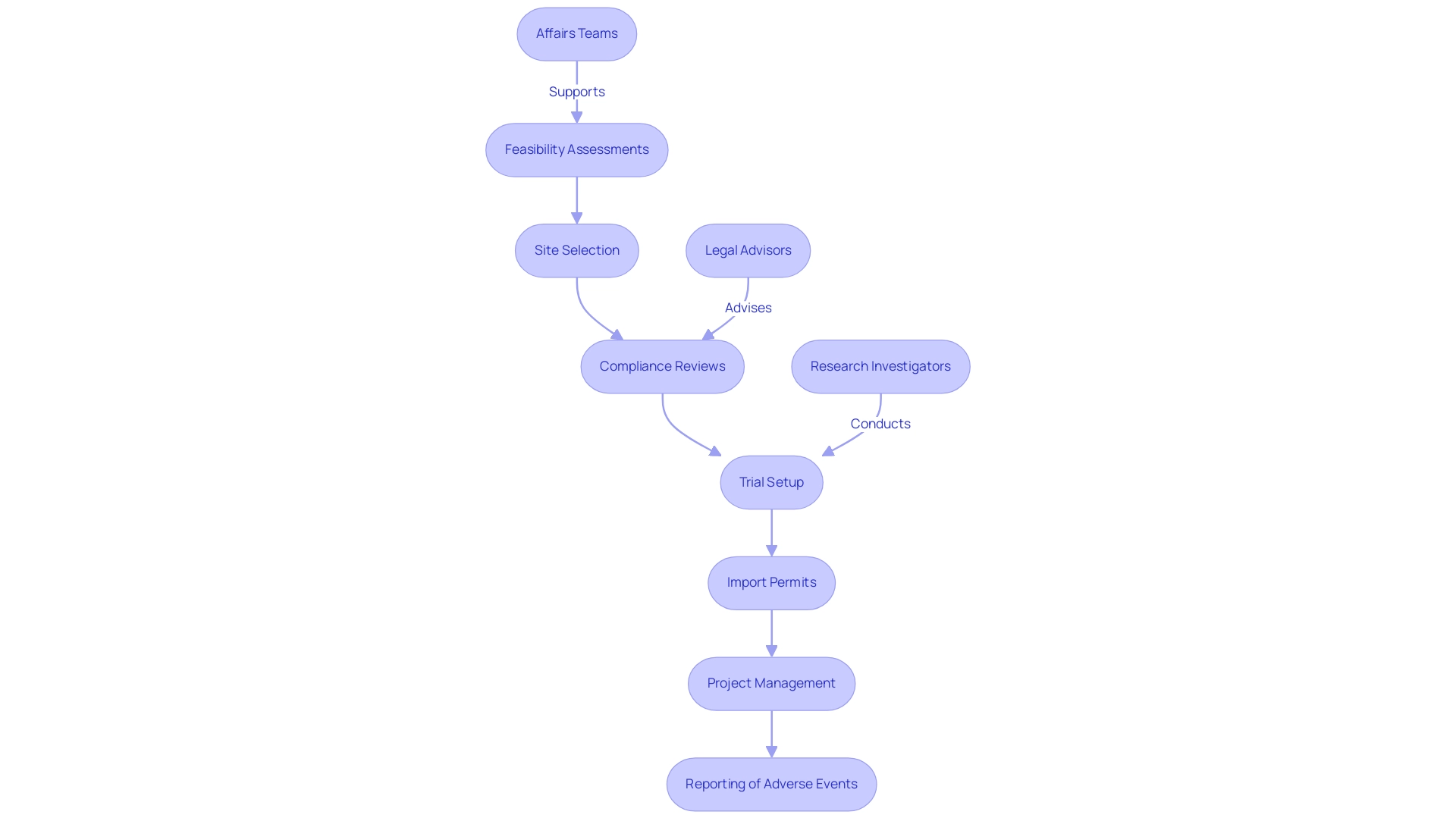
Navigating the regulatory affairs and clinical trials landscape for studies in Latin America involves a comprehensive understanding of various submissions, including Investigational New Drug (IND) applications and Clinical Study Applications (CSAs). Our service capabilities include:
- Feasibility assessments
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting of project status and adverse events
This encompasses a thorough review and feedback on research documents to ensure compliance with country requirements, as well as the import permit and nationalization of investigational devices.
Essential components of these submissions include:
- Meticulously detailed study protocols
- Informed consent documents
- Complete investigator information submitted to the relevant governing authorities
With the total expense for a new medication surpassing $1 billion, the financial consequences of these submissions are considerable. Compliance with established timelines is crucial—particularly in reporting adverse events and fulfilling ongoing obligations under Good Clinical Practice (GCP).
Successful compliance submissions require active cooperation among stakeholders, including:
- Affairs teams
- Legal advisors
- Research investigators
- Those involved in regulatory affairs and clinical trials
As Katherine Ruiz, a specialist in compliance affairs for medical devices and in vitro diagnostics in Colombia, emphasizes, 'performing the research and analyzing the data is critical to the success of these submissions.' This collective effort ensures that all required documents are not only accurate but also complete, facilitating the approval process and enhancing the likelihood of successful outcomes.
Challenges in Regulatory Affairs: Navigating Complexities
Maneuvering through the intricate environment of compliance matters in research studies poses many difficulties. Organizations face the task of managing differing requirements across jurisdictions in regulatory affairs and clinical trials, especially since many countries in Latin America may be new to overseeing clinical trials. This can result in differences in reporting processes, with only 10% of submissions receiving approval on the first attempt and less than 20% of biotech firms successfully navigating compliance pathways without external support.
The necessity for specialized expertise in regulatory affairs and clinical trials is clear, especially since miscommunication with oversight authorities can lead to expensive delays. For instance, in open-label research, missing visits related to treatment type can bias treatment effect estimates if not adequately addressed, underlining the importance of implementing mitigating strategies. Furthermore, fostering a culture of compliance awareness within research teams is crucial, necessitating adequate training on adherence matters.
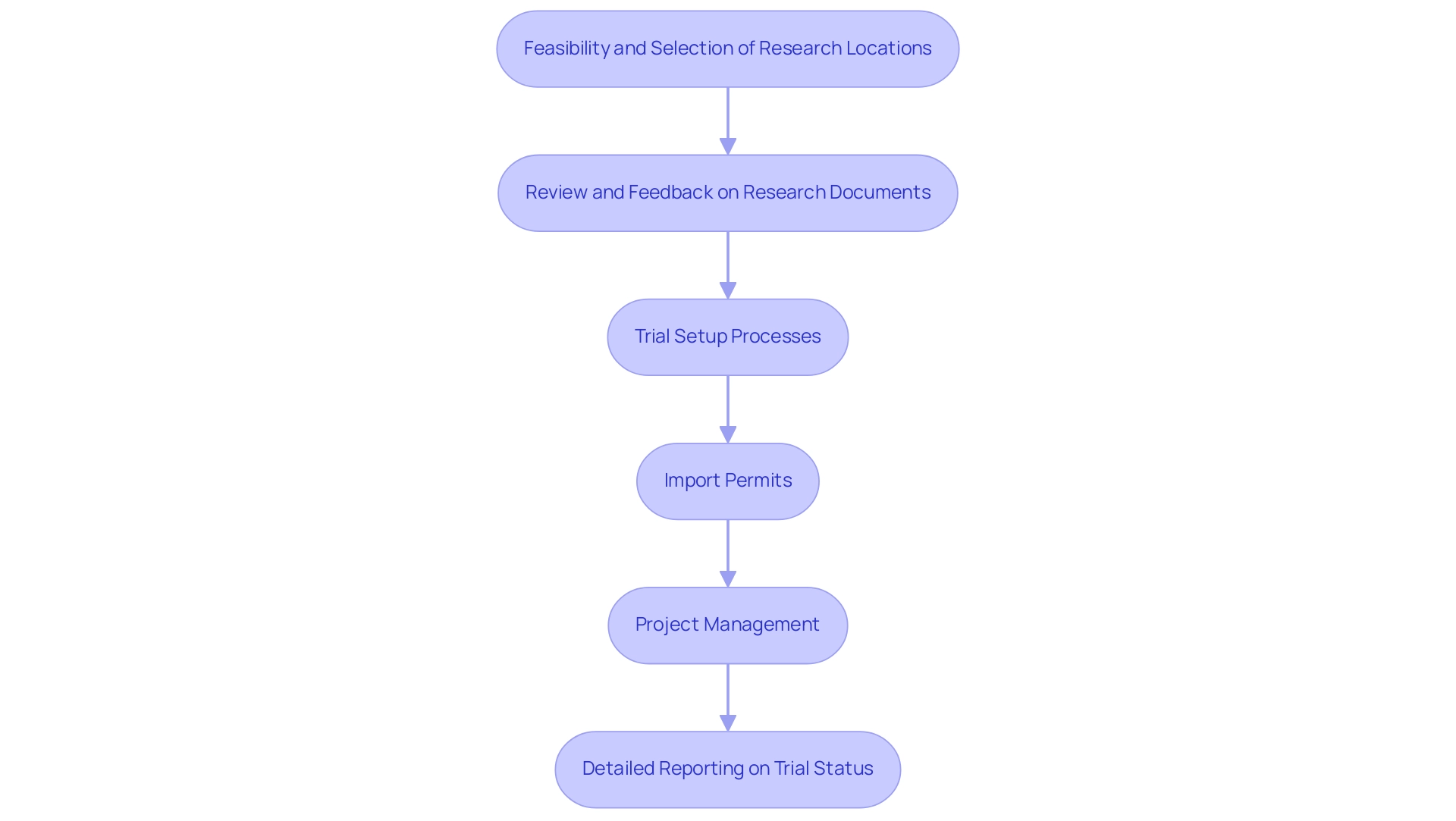
Our extensive trial management services encompass:
- Feasibility and selection of research locations and principal investigators
- Review and feedback on research documents to adhere to country requirements
- Trial setup processes including ethics committee and health ministry approvals
- Import permits
- Project management
- Detailed reporting on trial status, inventory, and serious and non-serious adverse events
The FDA highlights the necessity for IND/IDE applications in regulatory affairs and clinical trials for specific research studies to ensure scientific validity and safety, emphasizing the significance of compliance with regulations. Tackling these challenges requires efficient project management, effective communication abilities, and a proactive oversight strategy to guarantee the success of research initiatives.
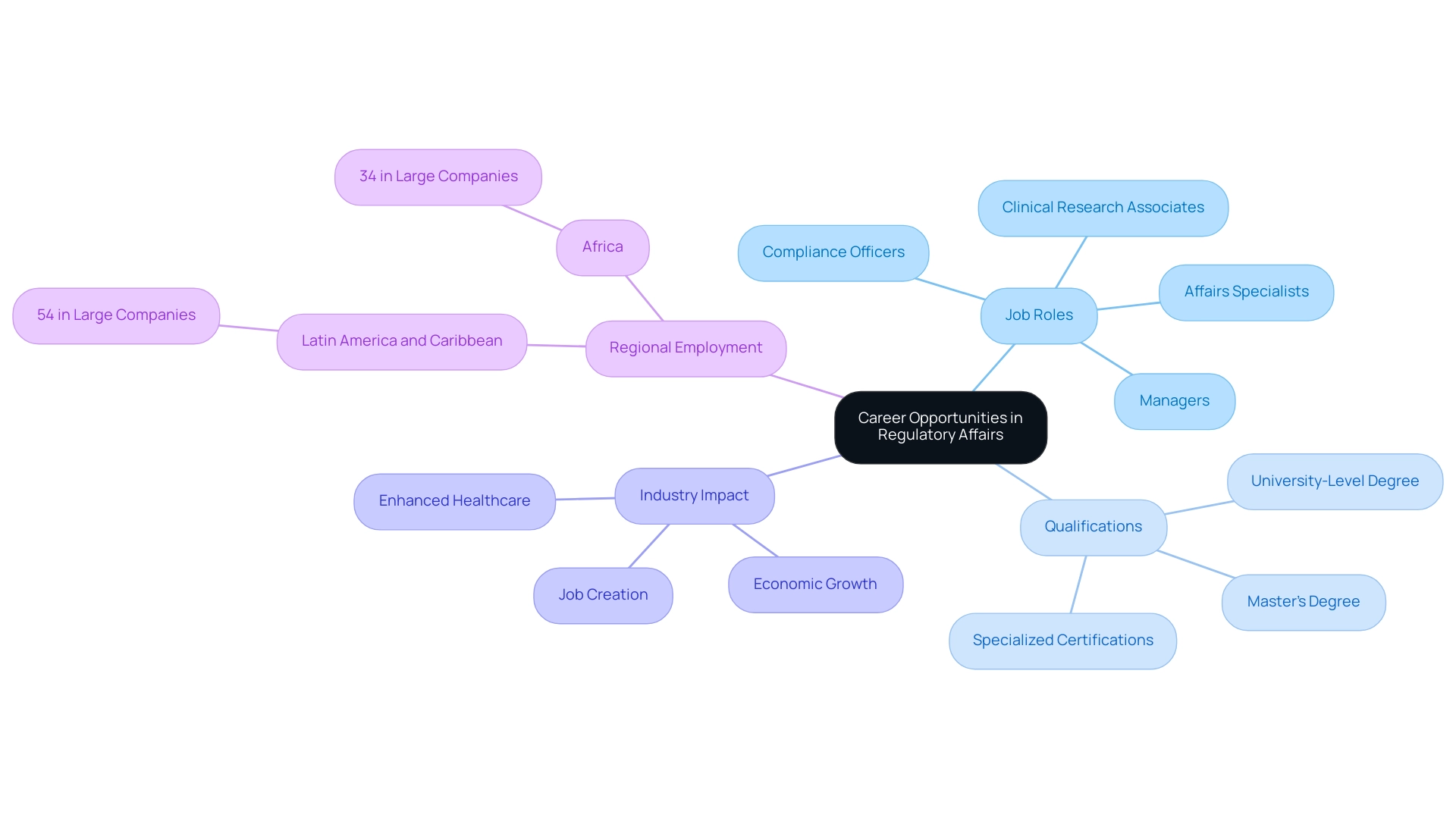
Career Opportunities in Regulatory Affairs: Pathways and Prospects
The landscape of career opportunities in compliance affairs is not only diverse but also expanding, reflecting the increasing demand for skilled professionals in this critical field. Positions vary from affairs specialists and managers to compliance officers and clinical research associates, each playing an essential role in ensuring adherence to standards. Notably, experts like Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, exemplify the caliber of professionals in this domain.
With a robust background in biomedical engineering, health economics, and cannabis oversight in Colombia, Ana previously held significant leadership roles at Colombia’s agency —INVIMA— where she contributed to shaping national policies. She also serves as a professor at top universities and an external consultant for global companies, showcasing the high level of expertise available in the region. According to a 2018 report by RAPS, over 99% of professionals in the field possess a university-level degree, with 44% holding a master’s degree, underscoring the academic foundation essential for success in these roles.
Usually, individuals seeking careers in compliance affairs come from backgrounds in life sciences, medicine, or related fields, along with a strong understanding of processes and submission requirements. Advanced degrees and specialized certifications in compliance matters significantly enhance job prospects and facilitate career advancement. As noted by Dennis Partners, who specialize in identifying and attracting world-class regulatory professionals, understanding candidate motivations and aspirations is crucial for long-term success in this field.
Furthermore, the influence of Medtech research on local economies creates ripples with extensive advantages, including job creation, economic growth, and enhanced healthcare, emphasizing the significance of this sector. Professionals in regulatory affairs and clinical trials play a crucial role in facilitating these impacts by ensuring that studies are conducted in compliance with local regulations, thereby fostering an environment conducive to economic development. As the clinical research environment continues to develop, professionals can explore various pathways, including opportunities in regulatory affairs and clinical trials, as well as consulting, pharmaceutical companies, and oversight agencies—each offering distinct challenges and rewards.
The current job market for compliance affairs professionals in 2024 is promising, with a notable increase in job openings driven by industry growth and the diversification of organization sizes. A case study reveals that in regions like Latin America and the Caribbean, 54% of regulatory professionals are employed by large companies, highlighting the regional differences in employment opportunities within Regulatory Affairs.
Conclusion
The discussion surrounding regulatory affairs in clinical trials highlights the essential role these frameworks play in safeguarding participant welfare and ensuring the integrity of research data. Understanding the foundations of Good Clinical Practice (GCP) and the functions of key regulatory bodies such as the FDA, EMA, and INVIMA is crucial for researchers. These organizations establish comprehensive regulations that dictate the design, conduct, and reporting of clinical trials, significantly influencing the approval processes for new therapeutics and medical devices.
Navigating the complex regulatory landscape presents various challenges, including differing requirements across jurisdictions and the necessity for specialized expertise. The importance of compliance cannot be overstated, as it directly impacts the success of clinical trials and the timely access to innovative treatments. Furthermore, fostering a culture of regulatory awareness within research teams is vital for overcoming these challenges and ensuring adherence to established standards.
As the demand for skilled professionals in regulatory affairs continues to grow, numerous career opportunities are emerging within this field. Individuals with backgrounds in life sciences, medicine, and related disciplines are well-positioned to contribute to this vital area of research. The impact of regulatory professionals extends beyond compliance; they play a pivotal role in economic development and public health by facilitating the introduction of groundbreaking medical innovations.
In conclusion, the evolution of regulatory affairs is not only fundamental to the integrity of clinical trials but also crucial for advancing public health and fostering innovation. As researchers and organizations navigate this intricate landscape, their commitment to regulatory compliance will ultimately shape the future of clinical research and improve outcomes for patients worldwide.
Frequently Asked Questions
What are regulatory affairs and clinical trials in research studies?
Regulatory affairs and clinical trials are essential for ensuring that investigations involving human participants comply with established policies and procedures, focusing on ethical and scientific quality standards.
What is Good Clinical Practice (GCP)?
Good Clinical Practice (GCP) encompasses a set of internationally recognized ethical and scientific quality standards crucial for safeguarding participant welfare and ensuring the integrity of research data.
What recent partnership has been established to enhance research studies in Latin America?
Bioaccess™ has partnered with Caribbean Health Group to establish Barranquilla as a premier location for research studies in Latin America, with support from Colombia's Minister of Health.
What is the significance of sharing results in interventional research initiatives?
As of May 2023, 94% of interventional research initiatives have shared their results, highlighting the importance of adherence to regulations and transparency in medical experiments.
Why is informed consent important in clinical trials?
Informed consent is vital as it empowers participants with the knowledge required to make informed decisions about their involvement in research studies.
What trial management services are essential for conducting ethical clinical trials?
Essential trial management services include feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting.
What role does INVIMA play in Colombia's regulatory framework?
INVIMA, as Colombia's National Food and Drug Surveillance Institute, oversees medical device regulation and the approval process for investigational products, ensuring adherence to health standards.
How do regulatory organizations like the FDA and EMA influence clinical trials?
The FDA and EMA supervise research studies, ensuring that new drugs and therapies are safe and effective prior to market entry, which impacts the authorization procedure for experimental products.
What are some recent developments in regulatory affairs and clinical trials?
The approval of OGSIVEO (nirogacestat) for treating progressing desmoid tumors on November 27, 2023, exemplifies the practical impact of regulatory frameworks on timely access to new therapies.
What services are offered to promote adherence to regulatory standards in clinical trials?
Services offered include feasibility studies, site selection, compliance assessments, testing setup, import permits, project management, and reporting to ensure compliance with industry standards.

