Overview
This article examines the regulatory pathways for medical device trials in Paraguay, underscoring their critical role in ensuring compliance, safety, and market entry for Medtech firms. It elaborates on the oversight provided by the Agencia Nacional de Vigilancia Sanitaria (ANVS), detailing how these pathways have significantly evolved since the enactment of Resolution 614. This evolution not only fosters innovation but also attracts foreign investment, all while addressing persistent challenges such as corruption within the approval process. The significance of these developments cannot be overstated, as they lay the groundwork for a more robust Medtech environment in Paraguay.
Introduction
Navigating the intricate landscape of medical device trials demands a profound understanding of the regulatory pathways that guarantee safety and efficacy prior to market entry. In Paraguay, the Agencia Nacional de Vigilancia Sanitaria (ANVS) serves a pivotal role in this process, shaping the trajectory for Medtech companies aspiring to introduce innovative technologies.
With a dynamic regulatory environment that fosters foreign investment and acknowledges the significance of diverse clinical trials, Paraguay emerges as a promising hub for medical advancements. As companies strive to align their strategies with the latest regulations, the implications of these pathways transcend mere compliance, influencing timelines, resource allocation, and ultimately, the success of clinical research initiatives.
This article explores the essential steps and considerations that delineate the regulatory process, underscoring the opportunities and challenges encountered by organizations in this ever-evolving field.
Define Regulatory Pathways for Medical Device Trials
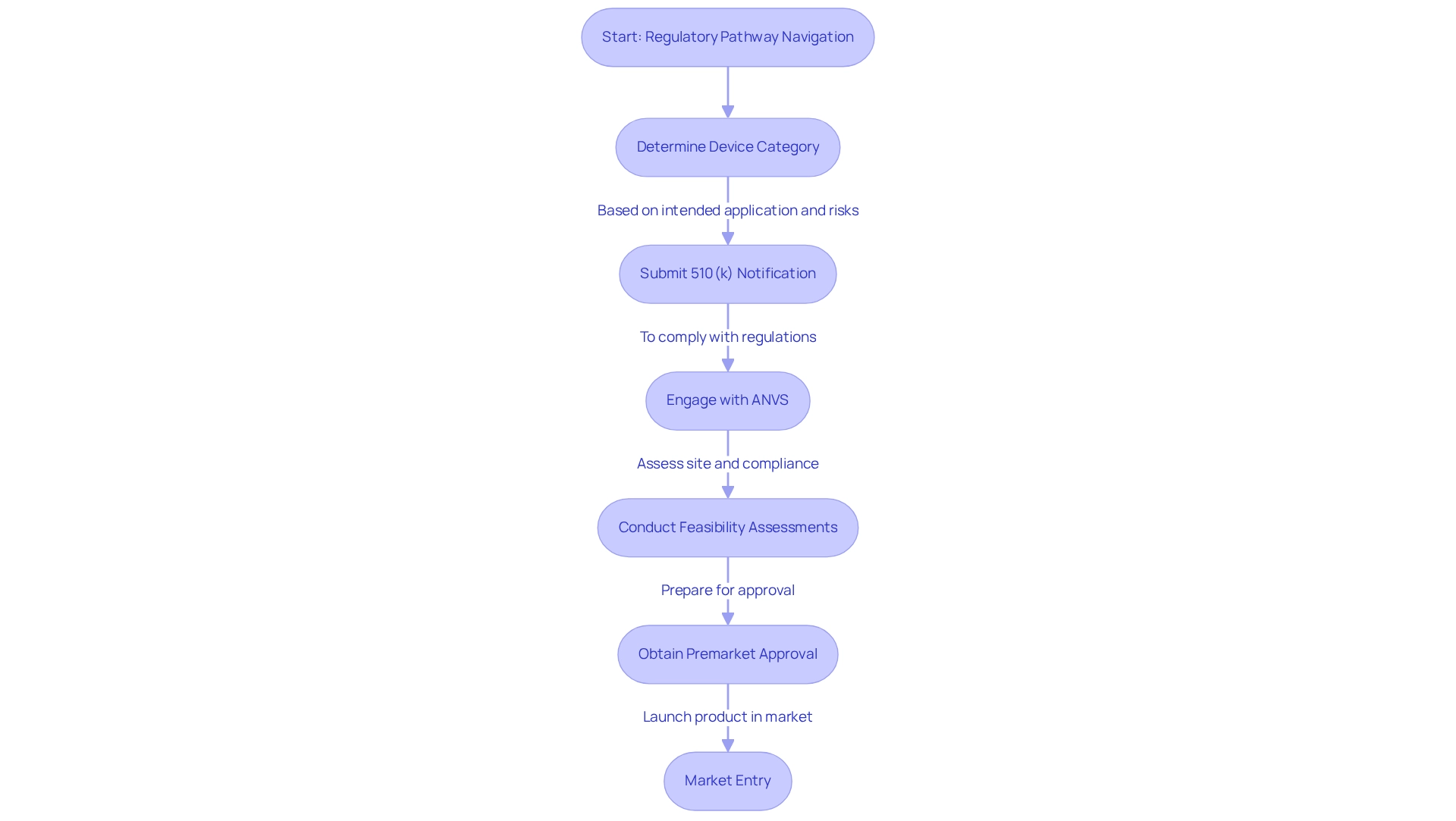
The structured processes known as regulatory pathways for device trials in Paraguay must be navigated by manufacturers to secure approval for their devices before entering the market. These pathways are essential in ensuring that devices adhere to the safety and efficacy standards established by governing bodies. In Paraguay, the Agencia Nacional de Vigilancia Sanitaria (ANVS) serves as the principal authority overseeing the regulatory pathways for device trials in Paraguay.
The categorization of a device, determined by its intended application and associated risks, significantly impacts the compliance standards it must fulfill. For instance, unclassified devices necessitate a 510(k) premarket notification submission to the Center for Devices and Radiological Health (CDRH), and understanding the regulatory pathways for device trials in Paraguay is crucial for Medtech companies aiming to navigate this intricate landscape and expedite the launch of their innovations to the market.
The regulatory pathways for device trials in Paraguay contribute to the country's evolving oversight environment, which is particularly favorable for promoting foreign investment and nurturing innovation, leading to successful device approvals. This trend is further bolstered by the increasing recognition of the need for research studies that encompass diverse populations, particularly Hispanics. Oversight structures can facilitate this inclusion by establishing standards that encourage diversity in research studies, ensuring that medical devices are developed with a broader demographic in mind.
As highlighted by industry experts, compliance professionals possess the skills to navigate regulations, yet the most adept among them understand the exceptions. This nuanced comprehension can unlock more opportunities than many realize, especially regarding premarket approvals (PMAs). With Colombia and Paraguay emerging as key players for early-feasibility studies (EFS) and first-in-human (FIH) trials, the region presents a unique opportunity for Medtech firms to utilize the regulatory pathways for device trials in Paraguay for successful market entry.
bioaccess® offers comprehensive management services for studies, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting—essential components for navigating these compliance pathways. The developing governance framework in Paraguay, as evidenced by the case study on the oversight environment for healthcare devices, is facilitating the entry of new participants into the market, thereby enhancing accessibility to advanced health technologies through regulatory pathways for device trials in Paraguay.
Explore the Regulatory Landscape in Paraguay
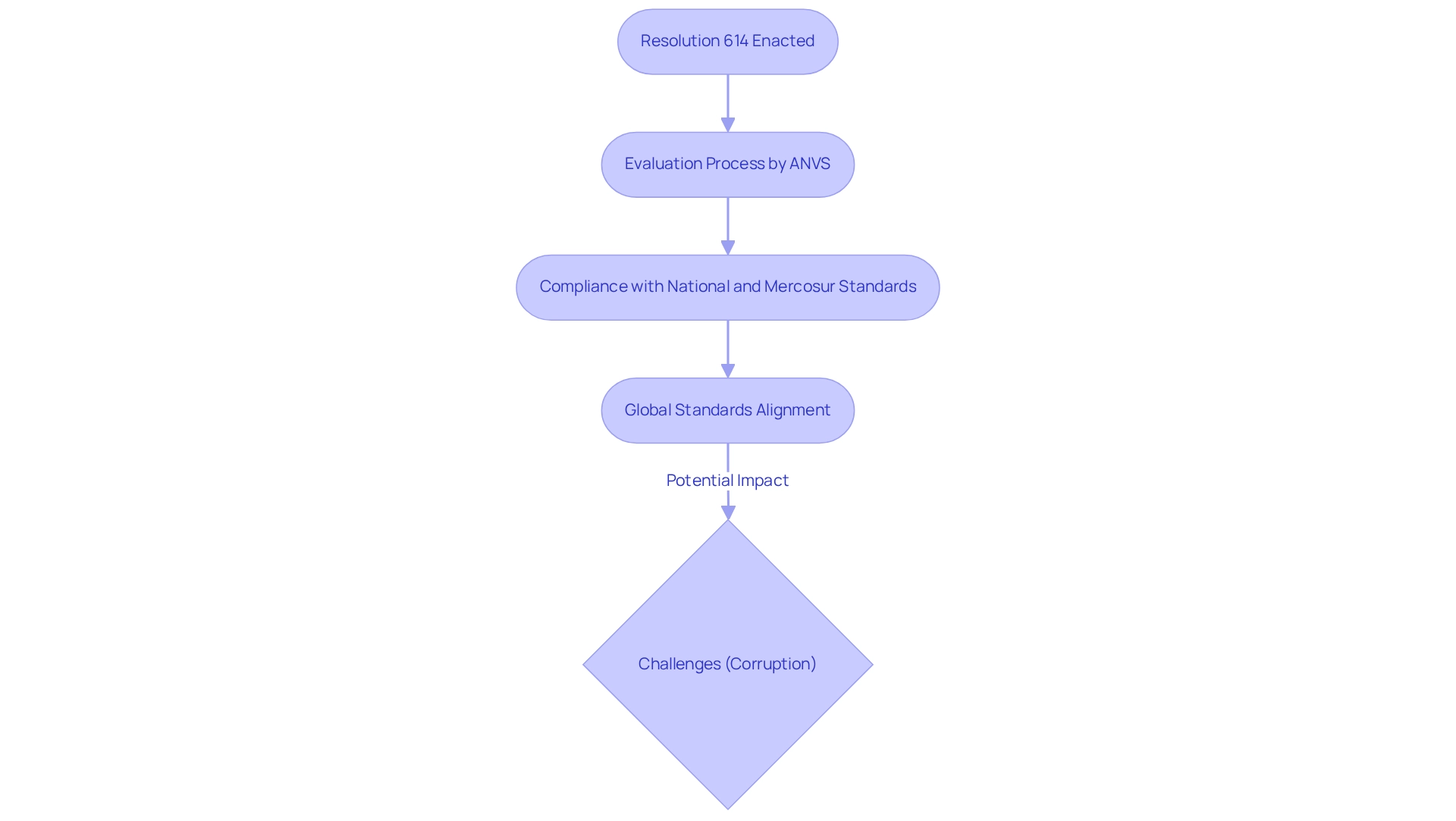
Since the enactment of Resolution 614 in December 2016, the regulatory pathways for device trials in Paraguay have experienced substantial transformation. This resolution established a comprehensive framework that the National Agency for Health Surveillance (ANVS) employs to regulate medical devices, incorporating the regulatory pathways for device trials in Paraguay to ensure compliance with both national and Mercosur standards.
The ANVS conducts a thorough evaluation process for research applications, which requires adherence to the regulatory pathways for device trials in Paraguay to demonstrate that devices are safe and effective for their intended purposes. As of 2025, the regulatory pathways for device trials in Paraguay are increasingly aligning with global standards, enhancing the country's appeal for Medtech firms seeking to conduct research in Latin America.
However, challenges such as corruption persist in Paraguay's research regulatory approval process, potentially influencing industry interest and funding. Despite these obstacles, the overall landscape presents promising opportunities for innovative healthcare technologies, particularly in response to the growing healthcare demands and technological advancements, which include understanding the regulatory pathways for device trials in Paraguay.
The healthcare device market in Paraguay is expanding, driven by rising demands, and presents abundant opportunities for local and international firms to introduce innovative health technologies tailored to the specific health challenges of the Paraguayan population, while navigating the regulatory pathways for device trials in Paraguay.
In this context, bioaccess® provides comprehensive clinical study management services, encompassing feasibility assessments, site selection, compliance reviews, study setup, import permits, project management, and reporting on study status and adverse events. Their expertise in overseeing Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies positions them as a vital ally in navigating the regulatory pathways for device trials in Paraguay.
Outline Key Steps in the Regulatory Process for Device Trials
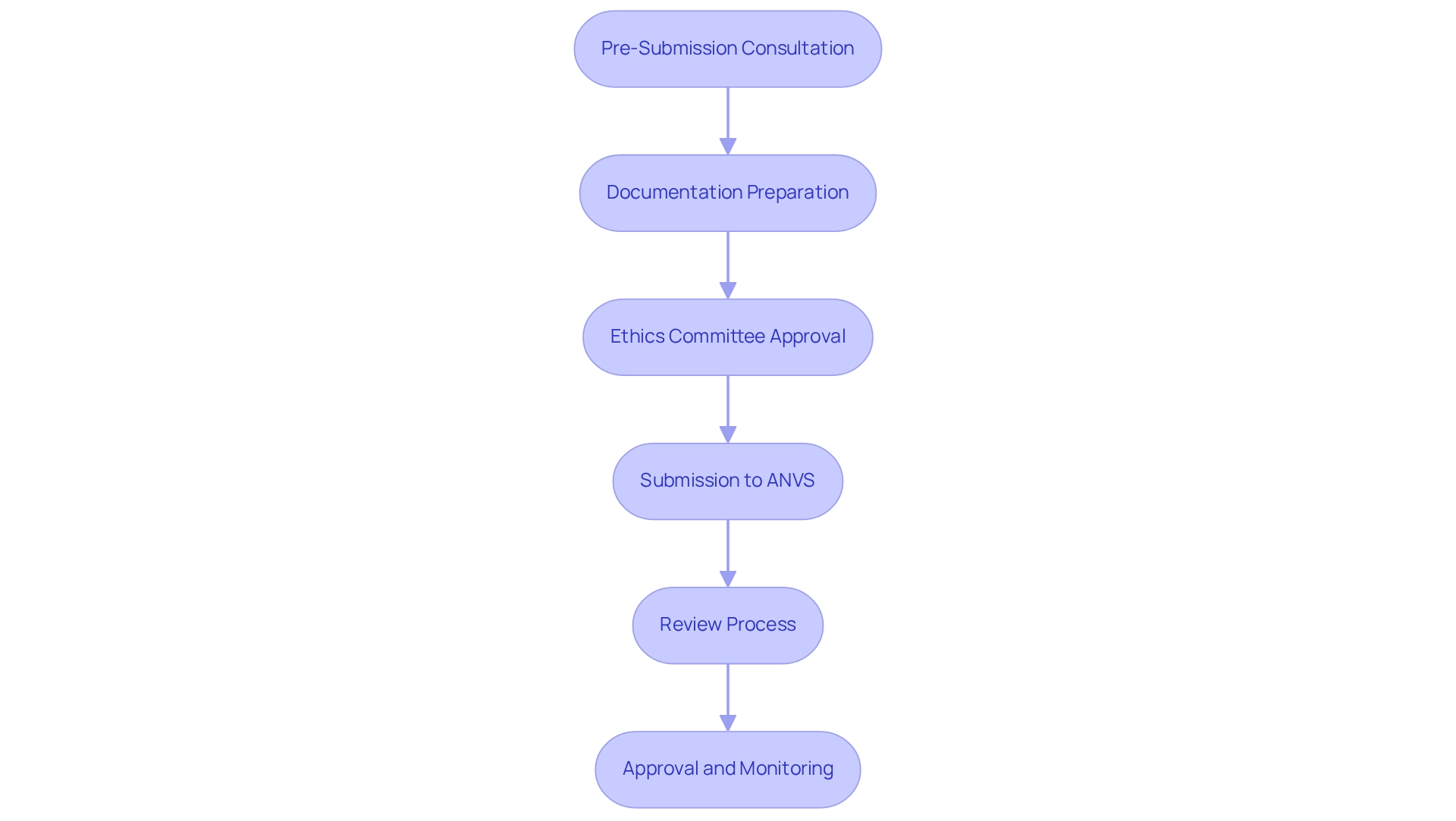
The regulatory process for medical device trials in Paraguay is part of the regulatory pathways for device trials in Paraguay, encompassing several critical steps designed to ensure compliance and safety.
- Pre-Submission Consultation: Engaging with the ANVS (Agencia Nacional de Vigilancia Sanitaria) early in the process is crucial. This step provides valuable insights into compliance expectations and can significantly streamline the approval process. As Dr. Mike Drues, a compliance consultant, observes, "Compliance professionals know the rules; the best ones understand the exceptions," emphasizing the significance of grasping compliance nuances. This proactive approach is essential for navigating the complexities of the Latin American medtech landscape, where local knowledge can greatly enhance market access strategies.
- Documentation Preparation: Companies must compile comprehensive documentation, which includes clinical study protocols, informed consent forms, and safety data. Thorough preparation at this stage is essential for a smooth review. This aligns with insights from Justin Bushko, who emphasizes the importance of user needs and iterative testing in MedTech startups, underscoring the need for purposeful prototyping in documentation. Ensuring that all documentation meets local regulatory standards is vital for maintaining compliance and fostering trust with stakeholders.
- Ethics Committee Approval: Before commencing studies, authorization from an Ethics Committee or Institutional Review Board (IRB) is essential. This guarantees that the research adheres to ethical guidelines and safeguards participant rights, a vital element of preserving client trust and ensuring the integrity of the clinical research process.
- Submission to ANVS: The complete application, encompassing all required documentation, is submitted to the ANVS for review. This submission signifies a crucial moment in the oversight journey, and understanding the regulatory pathways for device trials in Paraguay can facilitate smoother interactions with the ANVS.
- Review Process: The ANVS undertakes a meticulous evaluation of the application, which may include additional queries or requests for clarification. This thorough review is vital for maintaining high safety and efficacy standards. Companies should be prepared to respond promptly to any inquiries to avoid delays in the approval process.
- Approval and Monitoring: Upon approval, the trial can commence. However, ongoing monitoring and reporting of adverse events are required throughout the study to ensure participant safety and adherence to compliance standards. This ongoing supervision is crucial for maintaining the highest standards of patient care and adherence to regulations.
This organized method not only supports compliance with legal standards but also increases the chances of successful trial results. Importantly, substantial legislative changes for medical devices, particularly regarding regulatory pathways for device trials in Paraguay, are anticipated in 2024, making early involvement with the ANVS even more essential. Statistics indicate that such proactive regulatory strategies can lead to higher approval rates, underscoring their importance. Furthermore, bioaccess® is dedicated to ensuring information security and client trust throughout the research trial process, with established grievance and data protection procedures to address any concerns that may arise.
Discuss Implications of Regulatory Pathways on Clinical Research
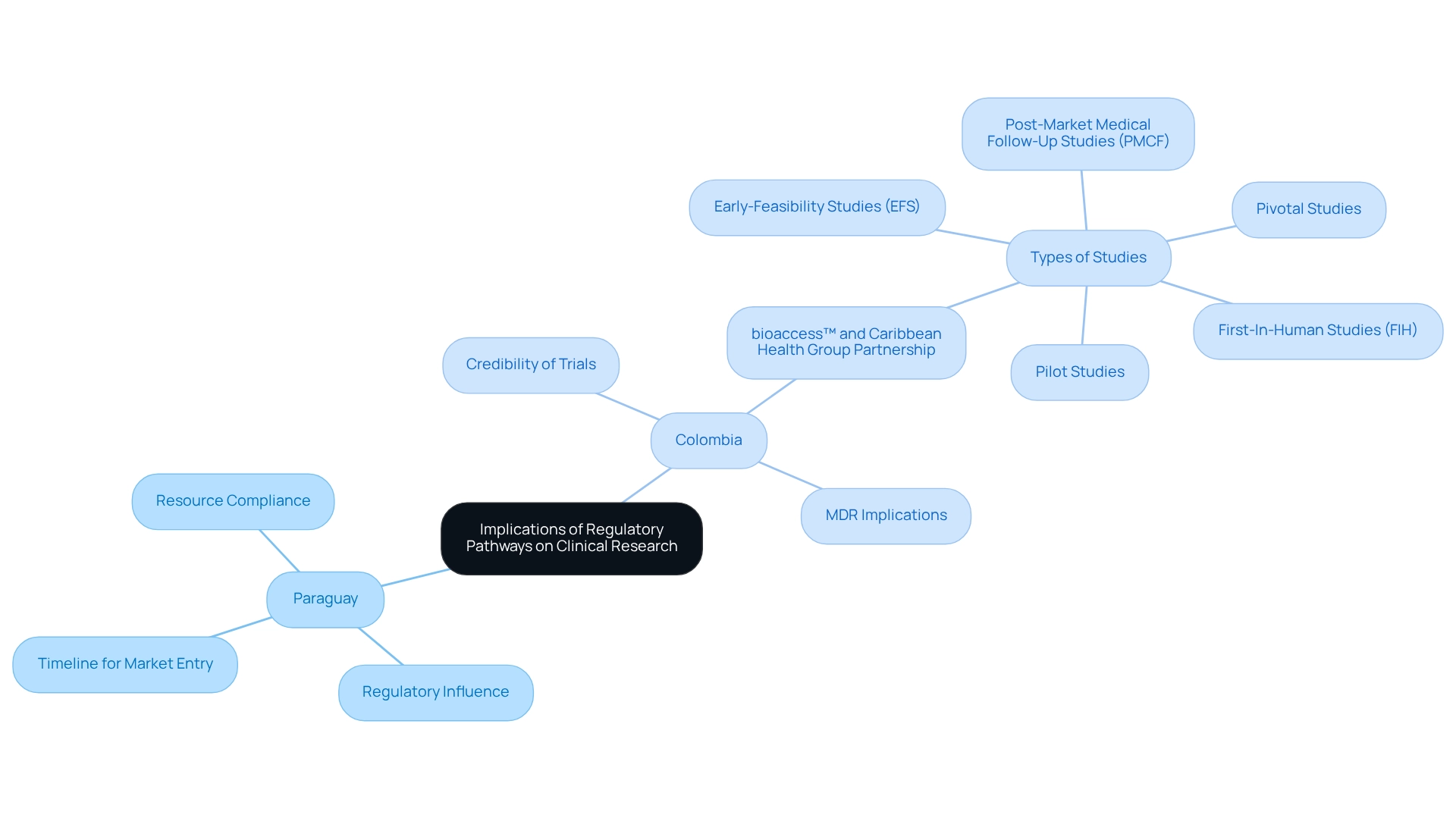
The regulatory pathways for device trials in Paraguay significantly influence medical research, determining both the timeline for medical device market entry and the resources necessary for compliance. A clearly defined regulatory framework can expedite approvals, fostering innovation and attracting investment within the Medtech sector. In contrast, overly stringent or ambiguous regulations can impede progress, resulting in delays and heightened costs.
In Colombia, the partnership between bioaccess™ and Caribbean Health Group seeks to establish Barranquilla as a premier location for medical studies in Latin America, backed by Colombia's Minister of Health. This initiative not only boosts the credibility of clinical trials carried out in the region but also fosters collaboration between local and international stakeholders, enhancing patient access to advanced healthcare technologies. Furthermore, the implementation of the Medical Device Regulation (MDR) restricts the principle of equivalence to a very limited number of cases, underscoring the importance of understanding these regulations. A regulated device market is crucial to guarantee the availability of safe and effective products, as emphasized in recent studies. By comprehensively understanding the regulatory pathways for device trials in Paraguay, companies can strategically navigate their research and development efforts, ensuring compliance while advancing medical knowledge and innovation.
For instance, bioaccess™ specializes in managing various medical studies, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Medical Follow-Up Studies (PMCF)
The collaboration with GlobalCare Clinical Trials has allowed bioaccess™ to improve ambulatory services for studies in Colombia, achieving over a 50% decrease in recruitment duration and 95% retention rates. This illustrates how regulatory pathways for device trials in Paraguay can enhance efficiency in medical studies and support local economic development through job creation and improved healthcare outcomes.
As Tuncay Bayrak noted, improving clinical trial capabilities is crucial for countries like Turkey, and similar enhancements in Colombia could further bolster its Medtech landscape.
Conclusion
Navigating the regulatory pathways for medical device trials in Paraguay presents both opportunities and challenges for Medtech companies. The Agencia Nacional de Vigilancia Sanitaria (ANVS) plays a crucial role in ensuring that devices meet safety and efficacy standards before market entry. With the regulatory landscape evolving significantly since the implementation of Resolution 614, Paraguay is increasingly aligned with international standards, making it an attractive location for clinical trials. However, companies must remain vigilant about potential obstacles, such as corruption, that may affect the approval process.
A structured approach to the regulatory process—beginning with pre-submission consultations and culminating in ongoing monitoring—can greatly enhance the likelihood of successful trial outcomes. By understanding the nuances of the regulatory environment, companies can streamline their documentation and engage effectively with the ANVS, which is essential for expediting approvals and fostering innovation. Furthermore, the emphasis on diverse clinical trial populations underscores the importance of inclusive practices in developing medical devices that cater to broader demographics.
Ultimately, the implications of regulatory pathways extend beyond mere compliance; they shape the entire landscape of clinical research and market entry strategies. As Medtech companies leverage the supportive regulatory environment in Paraguay, they stand poised to contribute to the advancement of medical technologies that address the unique health challenges faced by the population. Embracing these pathways not only accelerates innovation but also enhances the overall healthcare ecosystem, paving the way for a future where cutting-edge medical solutions are readily accessible.
Frequently Asked Questions
What are regulatory pathways for device trials in Paraguay?
Regulatory pathways are structured processes that manufacturers must navigate to secure approval for their devices before entering the market, ensuring adherence to safety and efficacy standards.
Who oversees the regulatory pathways for device trials in Paraguay?
The Agencia Nacional de Vigilancia Sanitaria (ANVS) is the principal authority overseeing the regulatory pathways for device trials in Paraguay.
How does the categorization of a device affect compliance standards in Paraguay?
The categorization of a device, based on its intended application and associated risks, significantly impacts the compliance standards it must fulfill. For instance, unclassified devices require a 510(k) premarket notification submission to the Center for Devices and Radiological Health (CDRH).
Why is understanding the regulatory pathways important for Medtech companies?
Understanding the regulatory pathways is crucial for Medtech companies to navigate the complex landscape and expedite the launch of their innovations to the market.
How do the regulatory pathways contribute to the investment and innovation environment in Paraguay?
The regulatory pathways support a favorable oversight environment that promotes foreign investment and nurtures innovation, leading to successful device approvals.
What is the significance of including diverse populations in research studies for medical devices?
Increasing recognition of the need for research studies that encompass diverse populations, particularly Hispanics, ensures that medical devices are developed with a broader demographic in mind.
What role do compliance professionals play in navigating regulations?
Compliance professionals are skilled at navigating regulations, and those with a nuanced understanding of exceptions can unlock more opportunities, particularly regarding premarket approvals (PMAs).
Why are Colombia and Paraguay considered key players for early-feasibility studies and first-in-human trials?
Colombia and Paraguay are emerging as significant locations for early-feasibility studies (EFS) and first-in-human (FIH) trials, providing unique opportunities for Medtech firms to utilize regulatory pathways for successful market entry.
What services does bioaccess® offer for navigating compliance pathways?
Bioaccess® offers comprehensive management services, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting.
How is the governance framework in Paraguay evolving to enhance market entry for new participants?
The developing governance framework in Paraguay is facilitating the entry of new participants into the market, enhancing accessibility to advanced health technologies through regulatory pathways for device trials.




