Introduction
In the realm of medical devices, shelf life testing emerges as a pivotal process that ensures the safety and efficacy of products intended for patient use. This intricate evaluation not only adheres to stringent regulatory frameworks established by authorities such as INVIMA but also addresses the multifaceted challenges posed by material degradation, microbial contamination, and varying environmental conditions.
As manufacturers strive to optimize device longevity, methodologies such as:
- Accelerated Shelf-Life Testing (ASLT)
- Real-time testing
have gained prominence, each offering unique insights into product performance over time.
Furthermore, the role of packaging cannot be understated, as it significantly influences the stability and integrity of medical devices throughout their lifecycle. This article delves into the essential aspects of shelf life testing, exploring key methodologies, regulatory considerations, and the critical interplay between packaging and environmental factors that shape the future of medical device safety and reliability.
Fundamentals of Shelf Life Testing for Medical Devices
Shelf life testing medical device is an essential procedure for assessing shelf stability in medical products, determining the period during which a product can dependably perform its intended role while ensuring user safety. This testing of the shelf life testing medical device holds significant weight in both the regulatory approval process and the practical application of these instruments in clinical environments, especially within the frameworks established by INVIMA, Colombia's regulatory authority recognized as a Level 4 health authority by PAHO/WHO. Comprehending the longevity of products includes various elements, such as material deterioration, microbial contamination, and environmental conditions, all of which can negatively impact a product's effectiveness and safety over time.
Experts emphasize the importance of evaluating which materials may be susceptible to degradation under specific storage conditions, as highlighted by plan, who noted, '... and which of these materials would you evaluate as being susceptible to degradation/decomposition under storage conditions?' By conducting shelf life testing medical device, healthcare providers can establish an adequate shelf life, confidently rely on the integrity of medical equipment, ultimately safeguarding patient health and ensuring compliance with INVIMA's regulatory standards. In 2020, the global market for shelf life testing medical devices was valued significantly, reflecting the increasing recognition of these factors.
Manufacturers are increasingly adopting Accelerated Shelf-Life Testing (ASLT) to meet ISO standards, facilitate risk assessment, and reduce costs. For instance, ASLT enables manufacturers, like those associated with bioaccess®, which has over 20 years of experience in Medtech, to shorten evaluation timelines, circumvent lengthy clinical trials, and expedite product market entry, thereby enhancing patient safety and product reliability while ensuring alignment with INVIMA’s oversight. Bioaccess® specializes in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).
A case study on the benefits of ASLT illustrates that by implementing this approach, manufacturers can effectively demonstrate compliance with regulatory standards while also addressing risks related to shelf life testing medical device. Katherine Ruiz, an expert in Regulatory Affairs for Medical Equipment and In Vitro Diagnostics in Colombia, emphasizes the significance of these processes in ensuring that medical products meet the necessary safety and efficacy standards.
Key Methodologies: Accelerated Aging vs. Real-Time Testing
Shelf life testing medical devices mainly employs two methodologies: accelerated aging and real-time assessment.
- Accelerated aging is a specialized procedure designed to estimate a product's useful lifespan by simulating long-term storage conditions in a condensed timeframe. This is achieved by exposing medical devices to elevated temperatures and humidity levels, which allows researchers to predict how the devices will perform over time.
- It is essential to note that when a product has a defined storage temperature range, the upper limit should be used to calculate the duration of accelerated aging. Furthermore, a minimum of two shelf-life time points is advised to guarantee thorough evaluation. A relevant case study, titled "Evaluation of Test Samples Post-Accelerated Aging," demonstrates the practical outcomes of this methodology, wherein the physical qualities and sterile integrity of test samples are compared to those not exposed to aging conditions, referred to as Zero-Time.
This case study generates a comprehensive report outlining the aging conditions, evaluation standards, and results, including statistical analysis of the performance of the sterile barrier system.
- In contrast, real-time assessment involves storing devices under standard conditions and periodically evaluating their performance and safety as part of shelf life testing medical device over the actual shelf life. While accelerated aging can provide quicker insights and is often favored for its efficiency, real-time evaluation offers a more direct observation of how products age in real-world conditions.
Each methodology has its own benefits and drawbacks; for example, accelerated aging can produce quicker outcomes but may not completely mimic real-world aging processes, whereas real-time evaluation is more indicative of actual product performance but necessitates a longer timeline. Understanding these trade-offs is crucial for making informed decisions in clinical research, especially regarding shelf life testing medical device. As Susan Haigney observed concerning EMA's recommendations, regulatory factors have a crucial influence in deciding the suitable approach for durability evaluation.
Regulatory Framework for Shelf Life Testing in Medical Devices
The regulatory environment for storage duration testing is mainly influenced by important entities like the FDA in the United States and the European Medicines Agency (EMA) in Europe. These agencies provide detailed guidelines that outline the requirements for conducting longevity studies, including the types of tests necessary, acceptable methodologies, and standards for data reporting. Recent updates indicate that the FDA has agreed to clarify aspects of § 820.15 within § 820.3, enhancing the readability and interpretation of the Quality Management System Regulation (QMSR).
Compliance with these regulations is critical for obtaining marketing approval and ensuring the safety of medical devices for patient use. Industry specialists like Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, who formerly occupied executive positions at Colombia’s regulatory body INVIMA, and Katherine Ruiz, an authority in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, highlight the importance of thorough documentation in durability assessment processes. For example, the product label may instruct users to take 2 tablets up to 3 times a day, not to exceed 10 days, highlighting the importance of durability evaluation for consumer safety.
Manufacturers are obligated to document their processes for shelf life testing medical devices and any modifications throughout the product lifecycle. As industry expert D. Dunn notes in In-Use Stability Testing: What Data Are Required And When?, adherence to these guidelines is essential for regulatory compliance.
Furthermore, unique aging study designs, particularly for products made from biologic or thermally sensitive materials, necessitate tailored approaches. These studies often employ environmental chambers specifically designed for shelf life testing medical devices to simulate real-world conditions, ensuring that the data generated is both relevant and accurate. This diligence not only supports regulatory compliance but also reinforces the commitment to patient safety.
The Role of Packaging in Extending Shelf Life
Packaging serves as a crucial element in the preservation of medical equipment, extending beyond mere protection to ensuring their longevity and integrity. The choice of materials, seals, and barrier properties plays a crucial role in determining how effectively a product can resist environmental challenges such as moisture, oxygen, and light, which are known to contribute to degradation. By utilizing optimal packaging solutions, manufacturers can improve the stability of their products, ultimately resulting in a prolonged duration for storage.
It is crucial for producers to conduct thorough shelf life testing medical device studies as part of their packaging validation and durability testing protocols. These studies not only validate that the packaging complies with regulatory standards but also affirm its effectiveness in maintaining the functionality of the product throughout its intended duration, which is crucial for shelf life testing medical device. Based on batch information, the actual product durability can be assessed, emphasizing the necessity for statistical evaluation in establishing the longevity of medical equipment.
A pertinent case study titled 'Estimating Batch and Product Shelf Duration' discusses the methodology for estimating true shelf spans based on stability studies of production batches, highlighting the importance of shelf life testing medical device to establish a consensus on the definition of true product shelf duration. As highlighted by Plastic Ingenuity,
- 'Our innovative, scalable healthcare solutions enable you to bring drug therapies, medical equipment, and diagnostic products to the market quicker,'
emphasizing the essential role that effective packaging plays in speeding up market readiness.
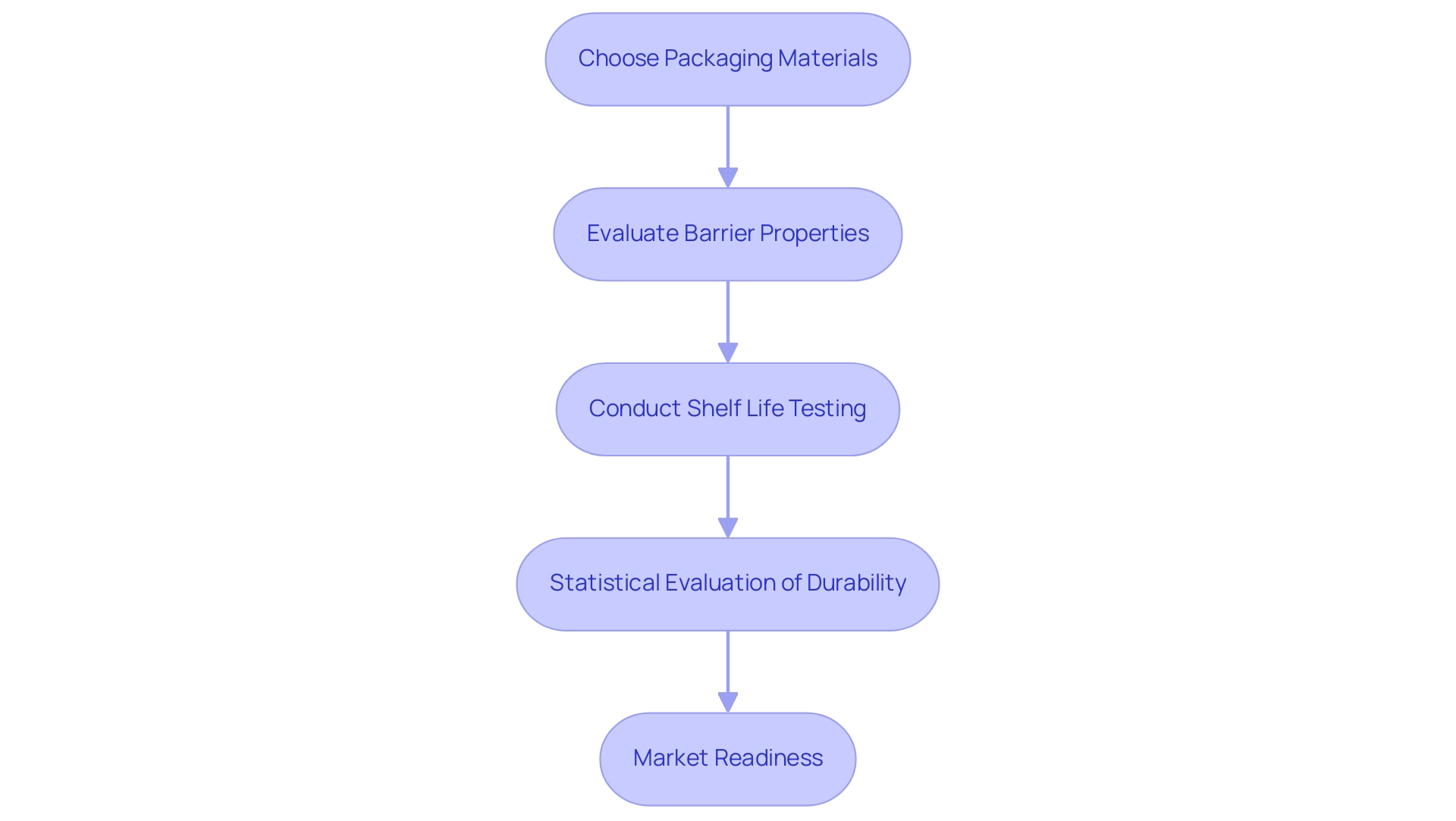
Challenges in Shelf Life Testing: Variability and Environmental Factors
The challenges of shelf life testing medical devices include the variability inherent in manufacturing processes and the significant impact of environmental factors on assessment outcomes. Fluctuations in temperature, humidity, and exposure to light can substantially affect the stability and performance observed during shelf life testing medical device evaluations over time. Variability between batches can arise from differences in material properties, leading to inconsistent results.
It is essential for researchers to meticulously design their studies, incorporating robust statistical methods to analyze data effectively. The integration of shelf life testing medical device evaluation with supply chain management systems is crucial, as it facilitates real-time monitoring and enhances product quality and safety. As Beaconpoint Labs notes, "Accurate food shelf-life evaluation ensures consumer safety, reduces food waste, and helps comply with regulatory standards."
Recent research has demonstrated that merging statistical evaluations offers a more reliable and accurate assessment of actual duration, with forecasts indicating an R-squared value of 85.22%. However, it is important to note that this combined statistical analysis does not outperform the separate analysis in all circumstances, underscoring the need for careful consideration of the methodologies used. The Arrhenius prediction case study exemplifies this approach, utilizing the Arrhenius equation to estimate degradation rates at standard storage temperatures based on accelerated testing at elevated temperatures.
This method indicated that the product could operate within specifications for up to 572 days at 25°C, suggesting a recommended duration of 561 days. Such meticulous consideration of environmental factors and robust analytical frameworks is vital in ensuring that shelf life testing medical devices yield determinations that are both accurate and reliable.
Conclusion
Shelf life testing is an indispensable element in the development and approval of medical devices, ensuring they maintain their safety and efficacy throughout their intended use. The methodologies discussed, particularly Accelerated Shelf-Life Testing (ASLT) and real-time testing, provide manufacturers with essential tools to assess product longevity while navigating the complex regulatory landscape set forth by authorities like INVIMA. These approaches not only streamline the testing process, allowing for quicker market entry, but also safeguard patient health by ensuring compliance with stringent standards.
The regulatory framework surrounding shelf life testing underscores the importance of rigorous documentation and adherence to established guidelines. As highlighted by industry experts, meticulous record-keeping and customized aging studies are crucial for meeting regulatory requirements, particularly for sensitive medical products. The role of packaging is equally critical, as it serves to protect devices from environmental factors that can compromise their integrity. Effective packaging solutions enhance device stability and extend shelf life, thereby reinforcing the commitment to patient safety.
However, challenges such as variability in manufacturing processes and environmental influences remain prevalent in shelf life testing. Addressing these issues through robust study design and statistical analysis is essential for producing reliable shelf life estimates. The integration of testing protocols with supply chain management further enhances product quality and safety.
In conclusion, the ongoing evolution of shelf life testing methodologies, regulatory compliance, and packaging innovations is vital for the future of medical device safety and reliability. As the industry continues to adapt to new challenges, the commitment to high standards in testing and product integrity will ultimately benefit both manufacturers and patients alike.
Frequently Asked Questions
What is shelf life testing for medical devices?
Shelf life testing for medical devices is a procedure that assesses the shelf stability of medical products, determining how long a product can reliably perform its intended role while ensuring user safety.
Why is shelf life testing important?
Shelf life testing is crucial for both regulatory approval and practical application in clinical settings. It helps ensure that medical devices remain effective and safe over time, considering factors like material deterioration, microbial contamination, and environmental conditions.
What regulatory authority oversees shelf life testing in Colombia?
INVIMA, Colombia's regulatory authority recognized as a Level 4 health authority by PAHO/WHO, oversees shelf life testing for medical devices.
What methodologies are used in shelf life testing for medical devices?
Two main methodologies are used: accelerated aging and real-time assessment. Accelerated aging simulates long-term storage conditions in a shortened timeframe, while real-time assessment involves storing devices under standard conditions and periodically evaluating their performance.
How does accelerated aging work?
Accelerated aging estimates a product's lifespan by exposing medical devices to elevated temperatures and humidity levels to simulate long-term storage conditions. This helps predict how devices will perform over time.
What are the advantages and disadvantages of accelerated aging?
The advantage of accelerated aging is that it provides quicker insights into product performance. However, it may not fully replicate real-world aging processes, which can be a limitation.
What is real-time assessment in shelf life testing?
Real-time assessment involves storing medical devices under standard conditions and periodically evaluating their performance and safety throughout the actual shelf life, providing direct observation of how products age in real-world conditions.
What are the trade-offs between accelerated aging and real-time assessment?
Accelerated aging offers quicker results but may not accurately mimic real-world aging, while real-time assessment is more indicative of actual performance but requires a longer evaluation period.
How are manufacturers adapting to shelf life testing requirements?
Manufacturers are increasingly adopting Accelerated Shelf-Life Testing (ASLT) to meet ISO standards, facilitate risk assessment, and reduce costs, thereby enhancing patient safety and product reliability.
What is the significance of case studies in shelf life testing?
Case studies, such as those demonstrating the outcomes of accelerated aging, provide practical insights into the methodologies used and help validate compliance with regulatory standards while addressing risks associated with shelf life testing.




