Overview
The article focuses on understanding the clinical research landscape in key Latin American countries, emphasizing the role of Contract Research Organizations (CROs) in enhancing research efficiency and effectiveness. It highlights how factors such as cost advantages, accelerated regulatory processes, and a robust healthcare system position countries like Colombia as attractive locations for clinical studies, while also addressing the challenges and emerging opportunities in the region's research environment.
Introduction
In recent years, Latin America has emerged as a vibrant hub for clinical research, driven by a confluence of competitive advantages and a growing commitment to advancing medical science. Contract Research Organizations (CROs) are at the forefront of this transformation, facilitating the planning, execution, and management of clinical trials across the region.
Countries like Colombia, Brazil, and Mexico are capitalizing on their unique demographics and healthcare systems to create a conducive environment for innovative research initiatives. With significant cost savings, expedited regulatory processes, and a robust patient recruitment framework, the landscape is ripe for exploration.
However, challenges such as regulatory complexities and funding limitations persist, necessitating strategic collaborations and a focus on local health issues. This article delves into the pivotal role of CROs in Latin America, highlighting current trends, regulatory challenges, and emerging opportunities that promise to reshape the future of clinical research in the region.
The Role of Contract Research Organizations in Latin America
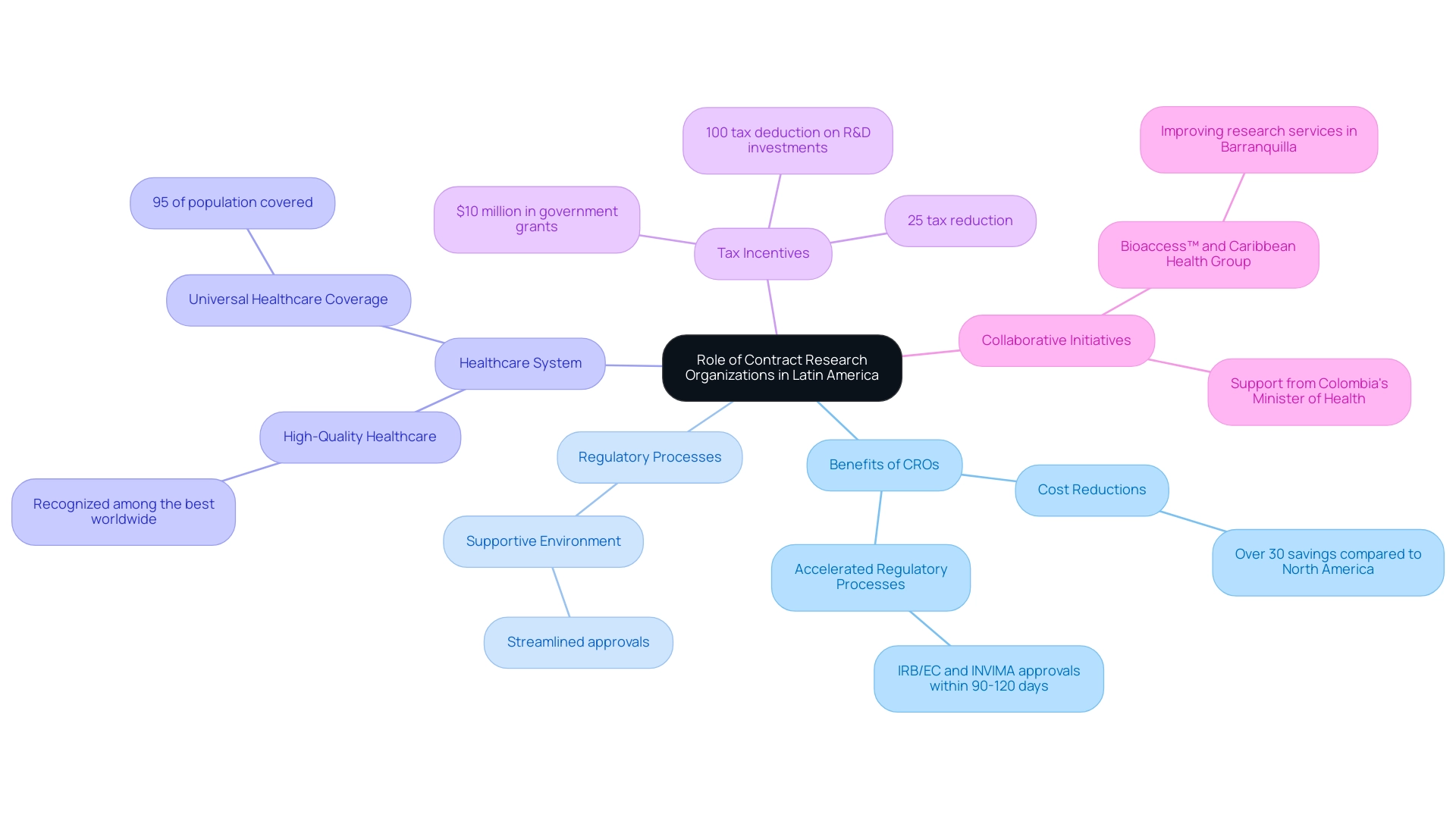
Contract Research Organizations (CROs) are crucial entities that provide a diverse range of services vital for the planning, execution, and management of research studies. In the setting of South America, especially Colombia, understanding the clinical research landscape in key Latin American countries highlights the essential role CROs play in utilizing the nation's competitive strengths for first-in-human studies. These encompass:
- Substantial cost reductions of over 30% relative to experiments in North America or Western Europe
- Accelerated regulatory processes with IRB/EC and INVIMA approvals usually finalized within 90-120 days
- A high-quality healthcare system recognized among the best worldwide
Furthermore, with a population exceeding 50 million and 95% covered by universal healthcare, patient recruitment is streamlined and effective, allowing for diverse participant pools. Joint initiatives, like the collaboration between bioaccess™ and Caribbean Health Group to improve research services in Barranquilla, are additionally backed by Colombia's Minister of Health, establishing the area as a prominent location for medical studies in South America. Furthermore, the area gains from considerable R&D tax incentives, featuring:
- A 100% tax deduction on investments in science, technology, and innovation initiatives
- A 25% tax reduction
- Approximately $10 million in government grants, which greatly promote investment in research studies
Understanding the clinical research landscape in key Latin American countries is essential as the CRO environment changes, greatly aiding in enhancing research efficiency and effectiveness, and positioning South America as a more competitive participant on the international scene. As Dr. Maria Gonzalez, a prominent investigator, stated, 'The financial incentives and supportive regulatory environment in Colombia make it an ideal location for conducting innovative research.
Current Landscape and Future Perspectives of Clinical Research in Latin America
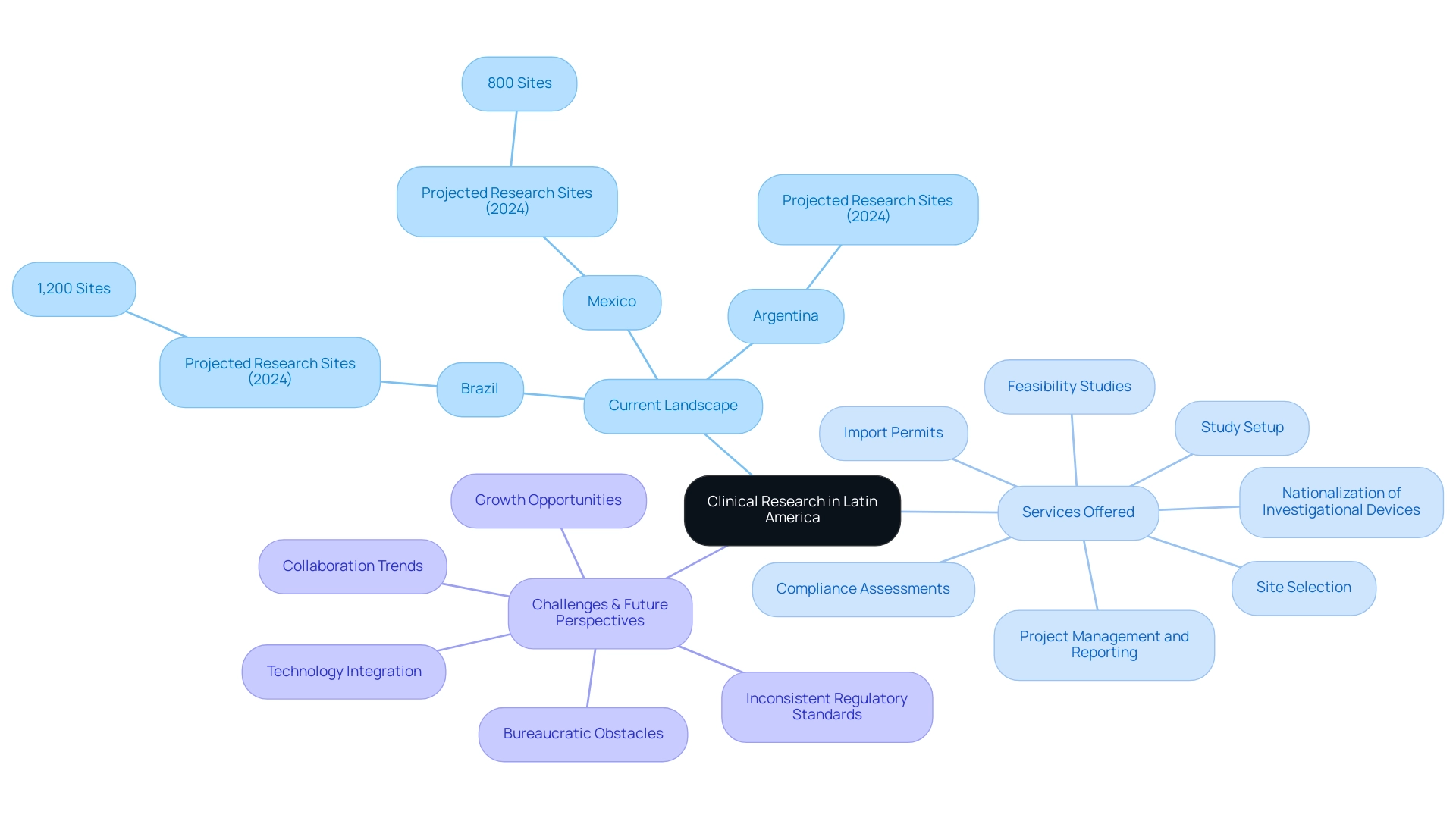
The medical investigation environment in Latin America is undergoing significant expansion, especially in Brazil, Mexico, and Argentina, which have created robust study infrastructures and a growing network of experimentation locations. As of 2024, Brazil is projected to host approximately 1,200 research sites, while Mexico anticipates around 800 locations, reflecting their commitment to advancing medical studies. Our extensive research management services include:
- Feasibility studies
- Site selection
- Compliance assessments
- Study setup
- Import permits
- Nationalization of investigational devices
- Thorough project management and reporting, encompassing study status and adverse event documentation
All created to navigate the intricacies of Regulatory Affairs in the region.
Despite these advancements, challenges persist, notably bureaucratic obstacles and inconsistent regulatory standards that can hinder progress. Insights from the case study titled 'Type Segment Analysis of South America Healthcare Contract Research Organization Market' reveal year-over-year growth rates in research studies, indicating a growing market appeal for stakeholders. Looking ahead, future perspectives suggest a burgeoning trend towards increased collaboration between local entities and international sponsors, fostering innovative projects.
Moreover, the incorporation of sophisticated technology and enhanced data management methods is expected to improve the effectiveness of research processes, ultimately aiding stakeholders throughout the region. As observed by Manjiri Kanhere, Senior Research Associate at Cognitive Market Research, 'The ability to analyze complex data sets and provide actionable insights will be essential in addressing these challenges and capturing growth opportunities in the research sector of South America.' This collaboration not only addresses unmet needs but also drives economic growth through job creation, healthcare improvement, and the promotion of international collaboration in the Medtech field.
Navigating Regulatory Challenges in Latin American Clinical Trials
Navigating the regulatory environment of research studies in South America necessitates understanding the clinical research landscape in key Latin American countries and the varied requirements unique to each nation. Regulations can vary significantly, impacting critical components of execution, including study design, ethical approvals, and patient consent processes. As funding in the research sector in Latin America’s Andean Region rises from $3-4 million to over $50 million each year, the significance of adherence has never been more evident.
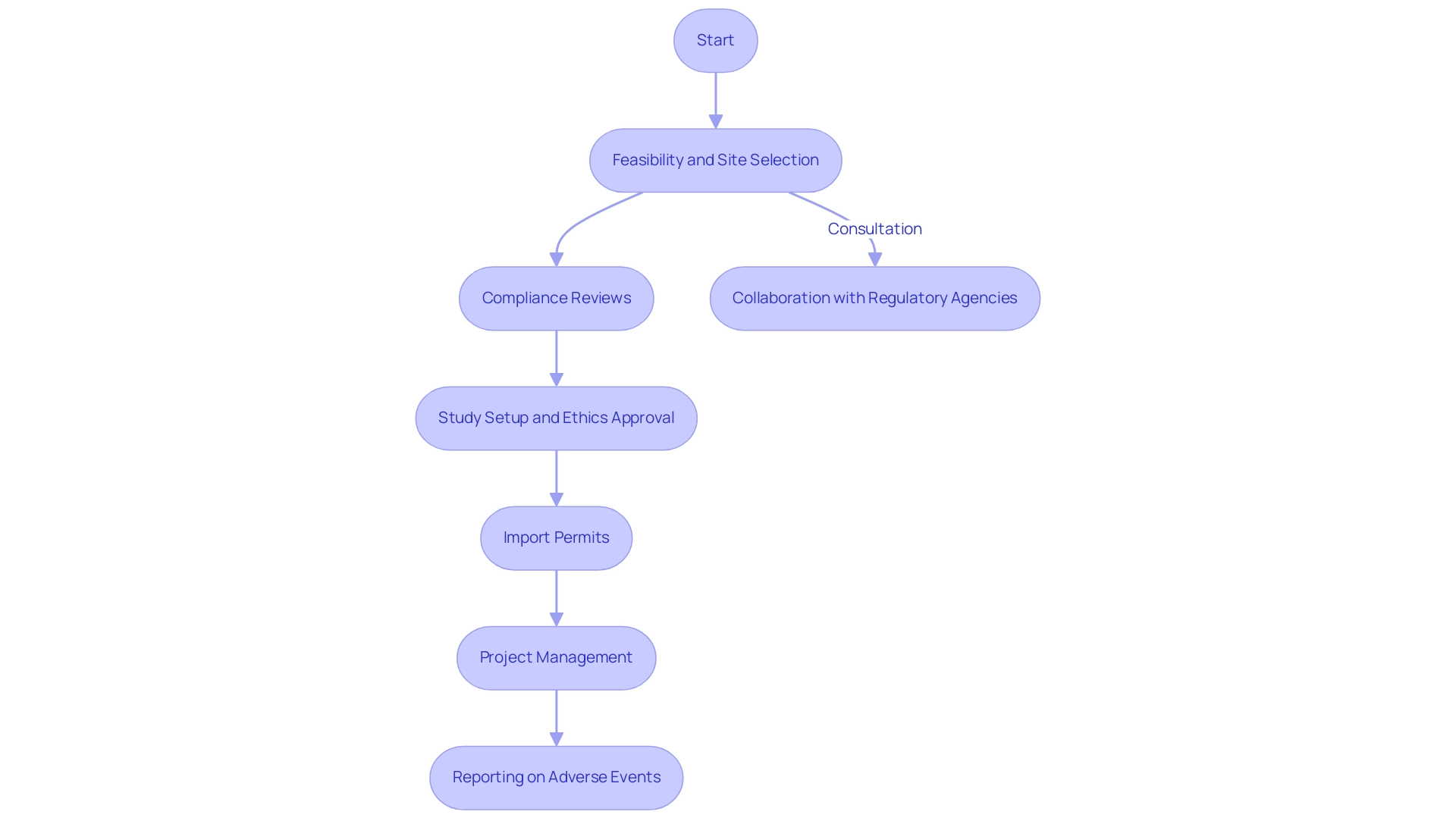
Our service capabilities encompass:
- Feasibility and selection of research sites and principal investigators
- Comprehensive compliance reviews
- Study setup and approvals from ethics committees and health ministries
- Import permits
- Meticulous project management
- Detailed reporting on study status, inventory, and both serious and non-serious adverse events
Experts like Ana Criado, Director of Regulatory Affairs with a robust background in biomedical engineering and health economics, and Katherine Ruiz, a specialist in Regulatory Affairs for medical devices, are instrumental in guiding researchers through these processes. Moreover, understanding the role of INVIMA, Colombia's National Food and Drug Surveillance Institute, is crucial as it serves as a Level 4 health authority by PAHO/WHO, overseeing medical device regulation.
Researchers are encouraged to foster close collaborations with local regulatory agencies and consider engaging consultants specializing in regional compliance to navigate these intricacies effectively. Integrating patient viewpoints into research funding proposals is also gaining recognition as a best practice, improving study relevance and acceptance. Staying updated on regulatory changes and emerging trends is vital for understanding the clinical research landscape in key Latin American countries, as it helps maintain compliance and mitigate risks.
For instance, the anticipated report on medical studies in Mexico for 2024 is expected to illuminate significant trends such as the rise of decentralized trials and the incorporation of digital health technologies, offering invaluable insights for researchers. Furthermore, aligning compliance strategies with case studies demonstrating successful navigation of regulatory challenges in South America will further empower trial directors in their pursuits.
Identifying Barriers to Clinical Research Development in Latin America
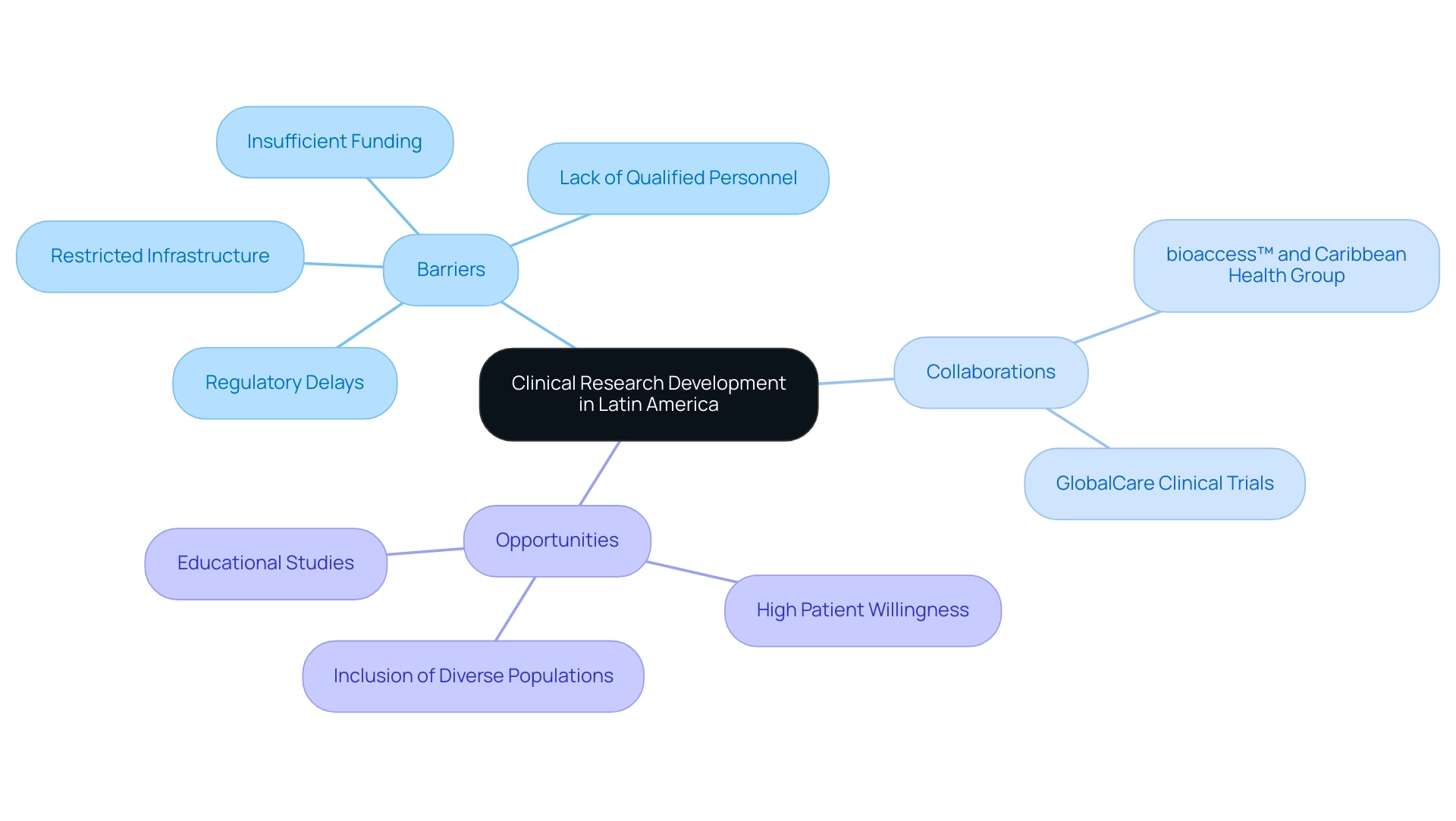
The advancement of medical studies in South America, especially in Colombia, encounters considerable obstacles like insufficient funding, restricted infrastructure, and a lack of qualified personnel. Although pharmaceutical firms mainly finance oncology studies in this area, the total investment is still inadequate, hindering the start and conclusion of vital investigations. In response to these challenges, a notable collaboration has emerged between bioaccess™ and the Caribbean Health Group, supported by Colombia's Minister of Health, Juan Pablo Uribe.
This initiative aims to position Barranquilla as the most appealing location for medical studies in Latin America, which is essential for understanding the clinical research landscape in key Latin American countries and enhancing the region's reputation in the global scientific landscape. Moreover, GlobalCare Clinical Trials has partnered with bioaccess™ to expand ambulatory services in Colombia, achieving over a 50% reduction in recruitment time and maintaining a remarkable 95% retention rate. Despite regulatory delays and low investment, understanding the clinical research landscape in key Latin American countries highlights opportunities for growth, especially considering the high willingness of patients to engage in studies.
This could be utilized to create educational studies addressing local health concerns, which are essential in closing the gap in understanding related to the application of experimental results to varied populations.
Tackling these obstacles necessitates cooperative endeavors among governments, educational institutions, and private sector participants, concentrating on improving investigative capabilities and workforce development. This coordinated strategy has the potential to establish a more beneficial environment for medical studies, contributing to economic growth and healthcare enhancement in the region. Moreover, media coverage from outlets such as Clinical Leader emphasizes the increasing interest in trials in this region, highlighting the significance of including the large, genetically diverse populations of this area in the global agenda.
Such media focus is essential for drawing additional funding and backing for health-related projects.
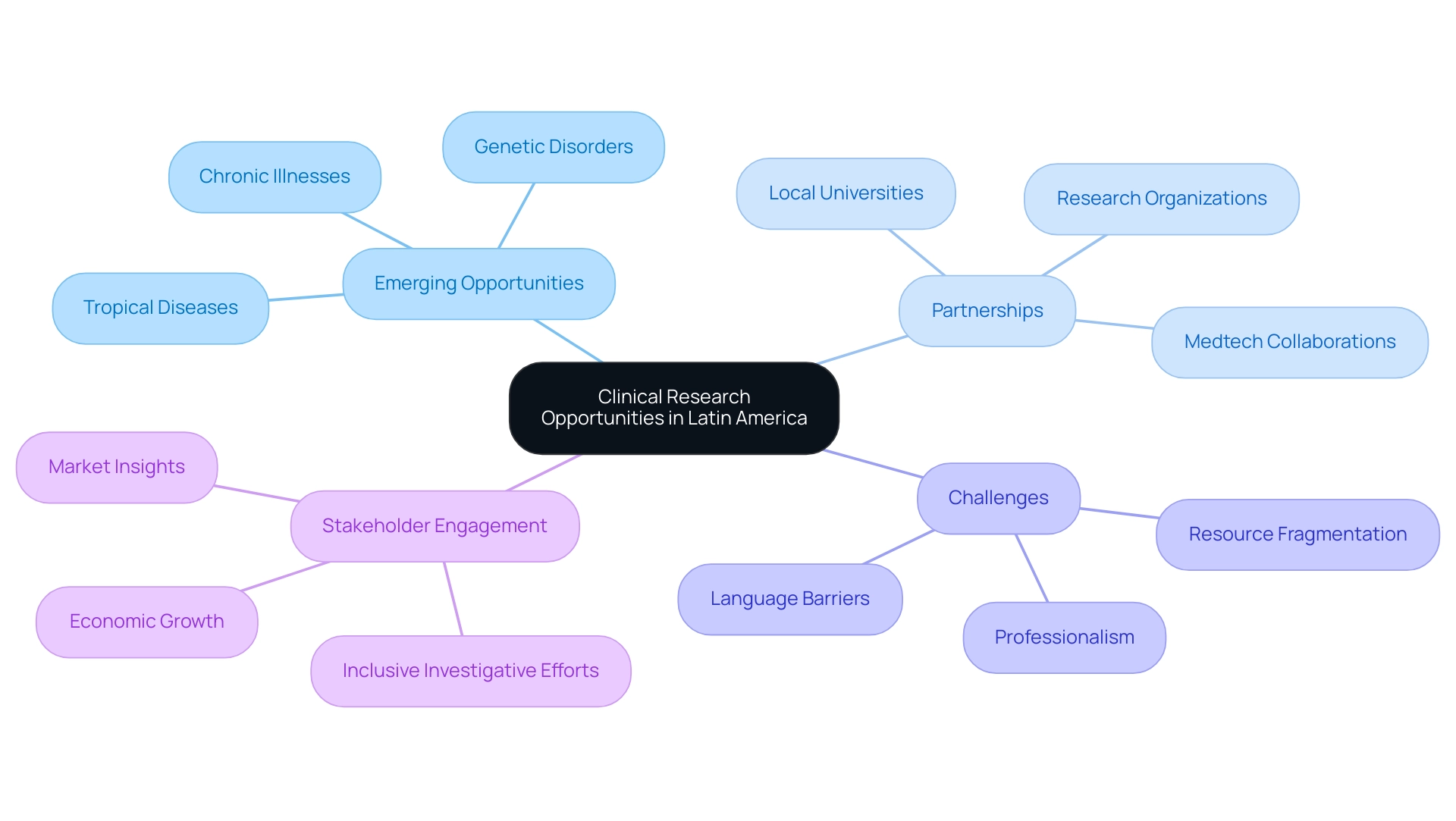
Emerging Opportunities for Clinical Research in Latin America
Understanding the clinical research landscape in key Latin American countries reveals a wealth of emerging possibilities for medical studies, largely due to its diverse populations and the prevalence of significant health issues. The region's focus on tropical diseases, genetic disorders, and chronic illnesses endemic to specific communities can provide invaluable insights that advance global health knowledge. Significantly, recent studies emphasize the urgent need for investigations aimed at tropical diseases, which persist in affecting the public health landscape.
To connect the gaps in medical research and innovation, partnerships with local universities and research organizations are crucial for understanding the clinical research landscape in key Latin American countries, which greatly improves the scope and effectiveness of studies through regional expertise and resources. However, US Medtech companies face several challenges in this landscape, including professionalism, language barriers, and fragmentation of resources, which can impede effective communication and collaboration with hospitals in South America. Furthermore, extensive research management services, such as feasibility studies, compliance evaluations, study setup, and project oversight, are vital for addressing these challenges.
As Medtech firms like bioaccess™ collaborate with entities such as Greenlight Guru, they are speeding up innovations and clinical evaluations in South America, illustrated by PAVmed's first-in-human study in Colombia. This collaborative effort not only enables advancements in medical studies but also contributes to improved patient outcomes across the region. As global sponsors increasingly acknowledge the potential of America, understanding the clinical research landscape in key Latin American countries becomes essential in the strategic pursuit of trial opportunities.
According to Perri Kasen, Senior Manager at Ernst & Young LLP, 'The vision of equitable experience and outcomes for all is paramount,' emphasizing the significance of inclusive investigative efforts. Additionally, stakeholders can greatly benefit from subscribing to the Horizon Databook, which provides access to over 1 million market statistics and 20,000+ reports, empowering them with insights into market trends and customer preferences. By strategically engaging in these opportunities, clinical research stakeholders can foster innovations that address the unique health challenges faced by Latin American populations, thus contributing to understanding the clinical research landscape in key Latin American countries while also driving economic growth and healthcare improvement in the region.
Conclusion
The evolution of clinical research in Latin America is marked by the pivotal role of Contract Research Organizations (CROs), which are instrumental in facilitating the execution and management of clinical trials. With countries like Colombia, Brazil, and Mexico at the forefront, the region offers significant advantages, including:
- Cost efficiencies
- Expedited regulatory processes
- A robust patient recruitment framework
These factors collectively position Latin America as a competitive player in the global clinical research landscape, driven by substantial investments and strategic collaborations aimed at enhancing research capabilities.
However, challenges remain, particularly regarding:
- Regulatory complexities
- Funding limitations that can impede progress
The necessity for strong partnerships among governments, academic institutions, and private stakeholders is crucial in overcoming these barriers. By fostering collaboration and focusing on local health issues, the region can develop innovative research initiatives that not only address unmet medical needs but also contribute to economic growth and healthcare advancements.
As the landscape of clinical research continues to evolve, the integration of advanced technologies and improved data management practices will further enhance the efficiency of trial processes. The growing recognition of Latin America as a critical hub for clinical research underscores the importance of inclusive and equitable research efforts. By strategically engaging with the diverse populations and unique health challenges present in the region, stakeholders can unlock new opportunities that not only benefit local communities but also enrich global health knowledge.
Frequently Asked Questions
What are Contract Research Organizations (CROs) and their role in research studies?
CROs are entities that provide a wide range of services essential for the planning, execution, and management of research studies, particularly in clinical research.
What advantages do CROs offer in South America, especially Colombia?
In Colombia, CROs offer substantial cost reductions (over 30% compared to North America or Western Europe), accelerated regulatory processes (IRB/EC and INVIMA approvals within 90-120 days), and access to a high-quality healthcare system.
How does the healthcare system in Colombia facilitate patient recruitment for clinical trials?
Colombia has a population of over 50 million, with 95% covered by universal healthcare, which streamlines and enhances patient recruitment, allowing for diverse participant pools.
What are some tax incentives available for research studies in Colombia?
Colombia offers a 100% tax deduction on investments in science, technology, and innovation, a 25% tax reduction, and approximately $10 million in government grants to promote research investments.
What is the current state of the medical investigation environment in Latin America?
The medical investigation environment is expanding, particularly in Brazil, Mexico, and Argentina, which have developed robust infrastructures for research. Brazil is projected to have about 1,200 research sites, while Mexico anticipates around 800 sites by 2024.
What services do CROs provide to manage research studies in Latin America?
CROs offer services including feasibility studies, site selection, compliance assessments, study setup, import permits, nationalization of investigational devices, and comprehensive project management and reporting.
What challenges do CROs face in Latin America?
CROs encounter bureaucratic obstacles and inconsistent regulatory standards that can impede progress in research studies.
What future trends are expected in the CRO landscape in South America?
There is an anticipated increase in collaboration between local entities and international sponsors, the incorporation of advanced technology, and improved data management methods to enhance research processes.
How does collaboration in the CRO sector benefit the economy?
Collaboration drives economic growth by creating jobs, improving healthcare, and promoting international partnerships in the Medtech field.

