Overview
The article focuses on the future of medtech trials in Latin America, highlighting the region's regulatory advantages, innovative partnerships, and the role of Clinical Research Organizations (CROs) in enhancing research capabilities. It emphasizes that countries like Colombia and Chile are emerging as key players due to their supportive regulatory environments and strategic investments, which are crucial for attracting clinical research and fostering innovation in the medtech sector.
Introduction
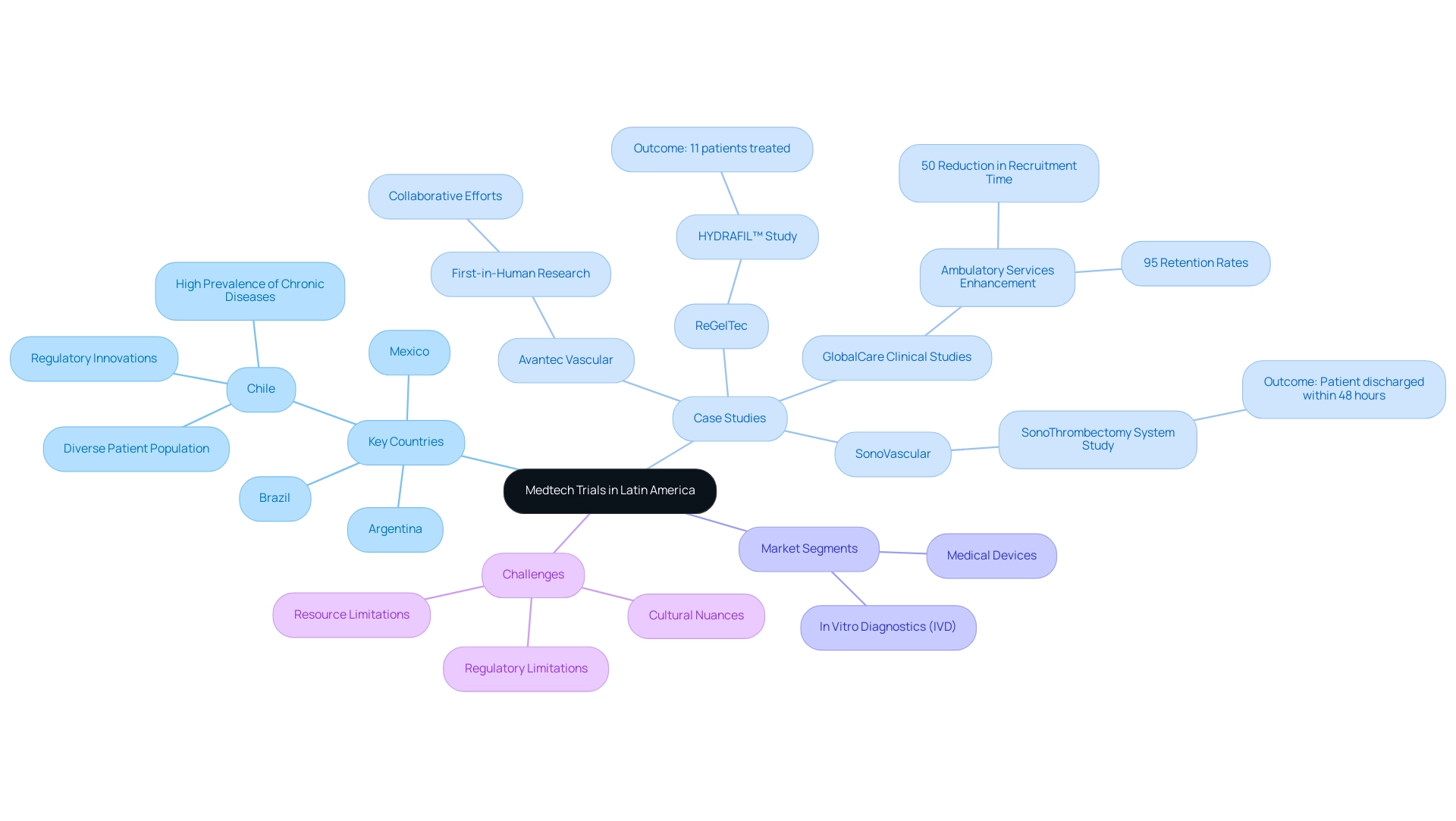
The Medtech trial landscape in Latin America is rapidly evolving, marked by a unique blend of regulatory advantages and a diverse patient population. Countries such as Brazil, Mexico, and Argentina are stepping into the spotlight, while Chile is making waves with its strategic investments and innovative regulatory approaches.
As the region becomes an increasingly attractive hub for clinical trials, organizations are navigating a complex web of regulations and cultural nuances to optimize their research efforts.
Highlighting recent successes and collaborative initiatives, this article delves into how Latin America is positioning itself as a key player in the global Medtech arena, paving the way for innovative therapies and improved healthcare outcomes.
Current Landscape of Medtech Trials in Latin America
The variety and swift development of the Medtech testing environment in the southern continent highlight the future of medtech trials in Latin America, with nations such as Brazil, Mexico, and Argentina rising as key contributors in this field. Regulatory benefits and a large patient base fuel this expansion, while Chile distinguishes itself through its strategic investments and regulatory advancements, positioning itself as a major influencer in the future of medtech trials in Latin America. As Julio G. Martinez-Clark, CEO of bioaccess, aptly notes, by combining regulatory speed, ethical oversight, and a robust healthcare system, Chile is not just participating in the medical device research landscape; it's actively shaping the future of medtech trials in Latin America.
Recent case studies exemplify this trend:
- ReGelTec's Early Feasibility Study on HYDRAFIL™ for chronic low back pain successfully treated eleven patients in Barranquilla, demonstrating the potential of innovative therapies in the region.
- Furthermore, Avantec Vascular's first-in-human research of an innovative vascular device, supported by bioaccess™ in regulatory submissions and site selection, highlights the collaborative efforts to position Barranquilla as a leading destination for medical studies, endorsed by Colombia's Minister of Health.
- 'GlobalCare Clinical Studies' partnership with bioaccess™ further enhances clinical study ambulatory services in Colombia, achieving over 50% reduction in recruitment time and impressive 95% retention rates.
The medical technology market here consists of two key segments: In Vitro Diagnostics (IVD) and Medical Devices, crucial for understanding the landscape. Investment in healthcare innovation continues to rise, reflecting a commitment to advancing the future of medtech trials in Latin America. However, challenges persist, as researchers must navigate diverse regulatory frameworks, cultural nuances, and resource limitations that can influence effectiveness.
For instance, the SonoThrombectomy System Study, initiated by SonoVascular, Inc. at Hospital DIPRECA, successfully removed a thrombus, with the patient discharged within 48 hours, asymptomatic and recovering well. Grasping these dynamics, including the regulatory hurdles and local healthcare infrastructure, is essential for optimizing study designs and ensuring successful outcomes, which is vital for the future of medtech trials in Latin America. The high occurrence of chronic illnesses further increases the appeal of the region for conducting clinical research, creating a rich environment for innovative studies and successful Medtech progress.
Navigating the Regulatory Landscape for Medtech Trials
The regulatory landscape for the future of medtech trials in Latin America presents a complex mosaic of regulations that can vary significantly from one country to another. Researchers must grasp the specific requirements for each location, including registration processes, ethical approvals, and post-market surveillance mandates. Recently, Colombia and Paraguay have emerged as pivotal locations for U.S. MedTech companies looking to conduct early feasibility studies (EFS) and first-in-human (FIH) evaluations, indicating the future of medtech trials in Latin America, with Colombia standing out for its competitive advantages.
Significantly, the Colombian R&D tax credit allows small and midsize businesses to claim a 50% tax credit on their R&D and innovation initiatives, providing a considerable financial incentive for conducting experiments in the country. Julio G. Martinez-Clark, CEO of Bioaccess®, underscores this shift, stating,
Colombia has recognized these benefits and has an ambitious science, technology, and innovation plan for 2022–2031 to become a knowledge economy.
Additionally, a case study titled 'Latin America as a Research Destination' emphasizes that the region supports around 10% of global medical studies, which underscores the future of medtech trials in Latin America, thanks to its ethnically diverse population and strong doctor-patient relationships that enhance its attractiveness for research.
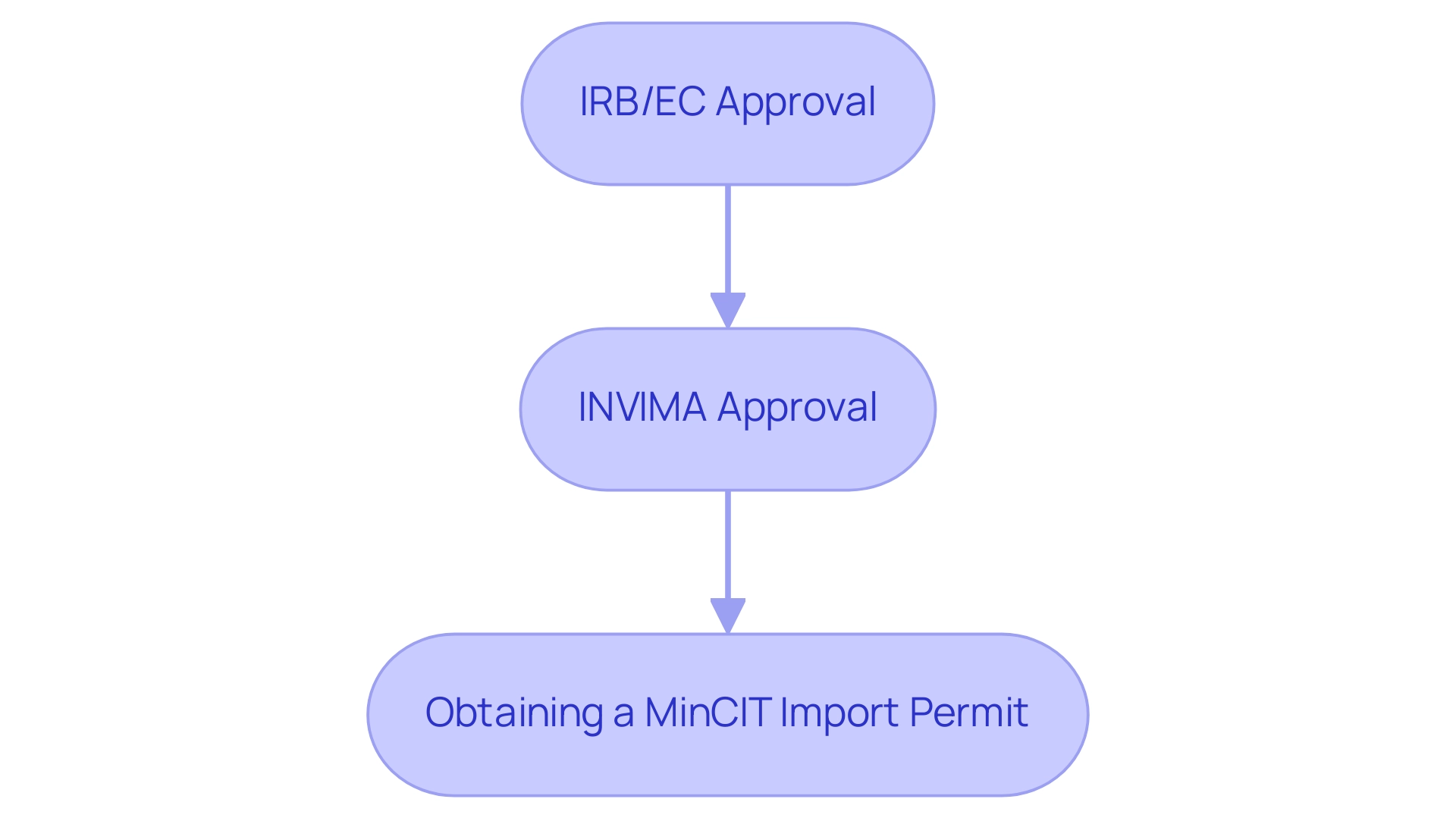
Engaging with local regulatory authorities early in project planning is not just advantageous but essential for navigating these regulatory complexities efficiently. The procedure for securing research study approval in Colombia involves several critical steps, including:
- IRB/EC approval
- INVIMA approval
- Obtaining a MinCIT import permit
Utilizing regulatory consultants, such as bioaccess®, can provide crucial support, ensuring compliance and facilitating a more expedited approval process.
Bioaccess® focuses on helping clients throughout the complete research process, from initial planning to final approvals, ensuring that all regulatory requirements are fulfilled. As regulations evolve, particularly with new frameworks anticipated for 2024, staying informed and connected with local experts will be vital for the future of medtech trials in Latin America in this dynamic landscape. The EU's stringent requirements and slow approval processes further emphasize why the region, especially Colombia, is becoming an increasingly desirable location for clinical trials.
Additionally, if you have any queries or concerns about the processing of your information, you may contact our Grievance Officer at IMH ASSETS CORP (doing business as 'bioaccess®'), 1200 Brickell Avenue, Suite 1950 #1034, email: info@bioaccessla.com. We will address your concerns in accordance with applicable law.
Fostering Collaboration: US-Latin American Medtech Partnerships
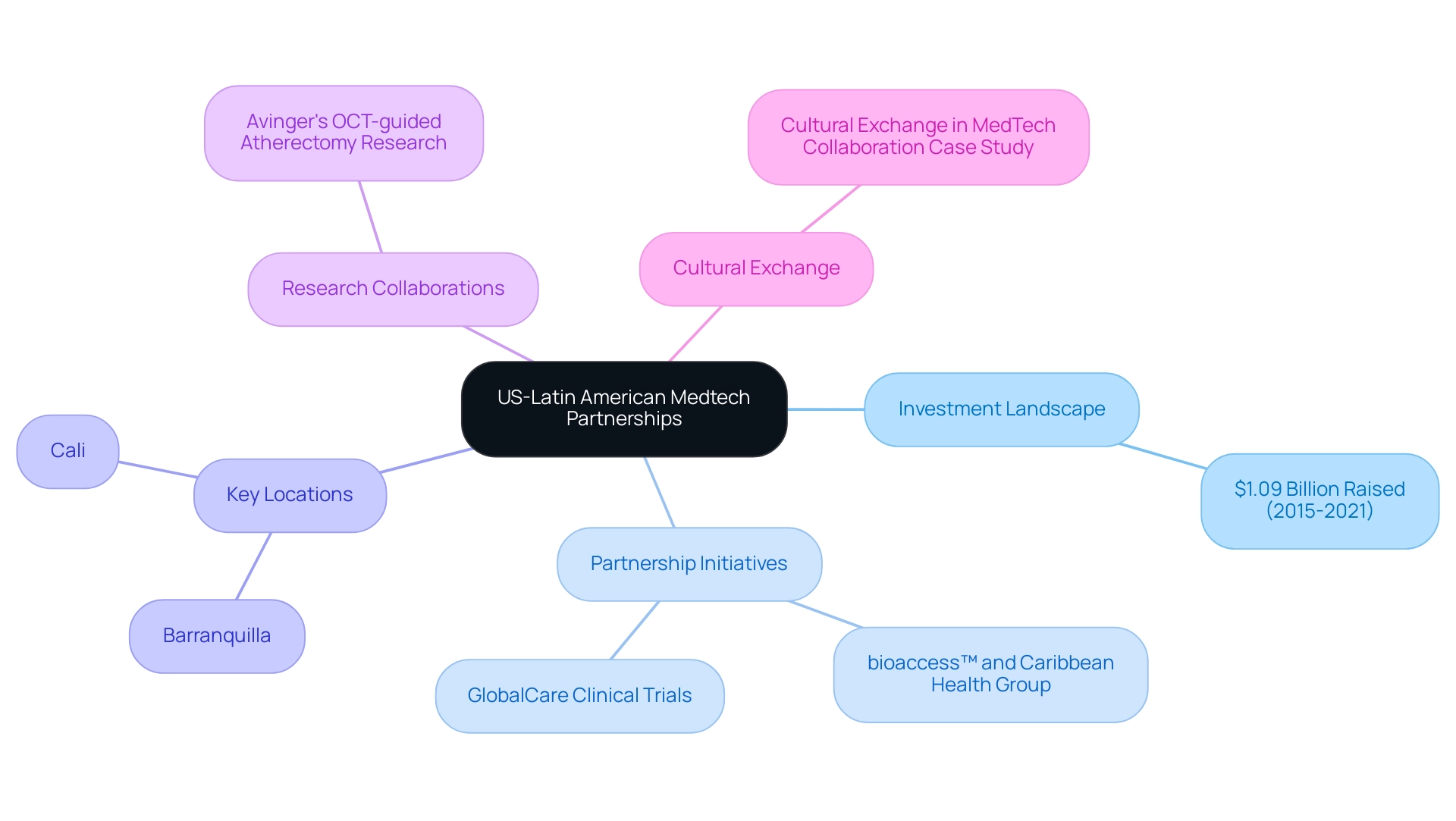
The expanding partnership between the US and South American Medtech sectors is becoming a cornerstone for fostering innovation and enhancing research capabilities, ultimately shaping the future of medtech trials in Latin America. From 2015 to 2021, Healthtech startups in the region raised an impressive $1.09 billion, indicating a robust investment landscape that supports the development of new technologies and solutions. As per a GHI report derived from internal primary research encompassing around 90% of healthcare facilities in the southern continent, the future of medtech trials in Latin America will increasingly rely on initiatives such as partnerships, research collaborations, and funding opportunities, allowing stakeholders to access vital resources and simplify research processes.
Moreover, the partnership between bioaccess™ and Caribbean Health Group establishes Barranquilla as a prominent location for the future of medtech trials in Latin America. Backed by Colombia's Minister of Health, this initiative seeks to enhance the future of medtech trials in Latin America. Julio G. Martinez-Clark, CEO of Bioaccess, highlights these developments, stating,
Colombia has recognized these benefits and has an ambitious science, technology, and innovation plan for 2022–2031 to become a knowledge economy, which is essential for the future of medtech trials in Latin America.
Additionally, Dr. John B. Simpson's work on Avinger's OCT-guided atherectomy research in Cali showcases the potential of these partnerships, especially in the context of the future of medtech trials in Latin America, to improve patient outcomes through innovative techniques. 'GlobalCare Clinical Trials' partnership with bioaccess™ has resulted in more than a 50% decrease in recruitment duration and an outstanding 95% retention rate, underscoring the significance of efficient patient recruitment approaches essential for the future of medtech trials in Latin America. The significance of cultural exchange in enhancing the efficacy of partnerships is highlighted in the case study titled 'Cultural Exchange in MedTech Collaboration,' which emphasizes how fostering open dialogue and mutual respect can lead to stronger connections and improved healthcare outcomes.
As medical researchers, proactively seeking out partnerships with US Medtech firms, academic institutions, and non-profit organizations can leverage shared expertise to drive advancements in the field, which are crucial for the future of medtech trials in Latin America and ultimately contribute to improved healthcare outcomes across the region.
The Role of CROs in Advancing Medtech Trials
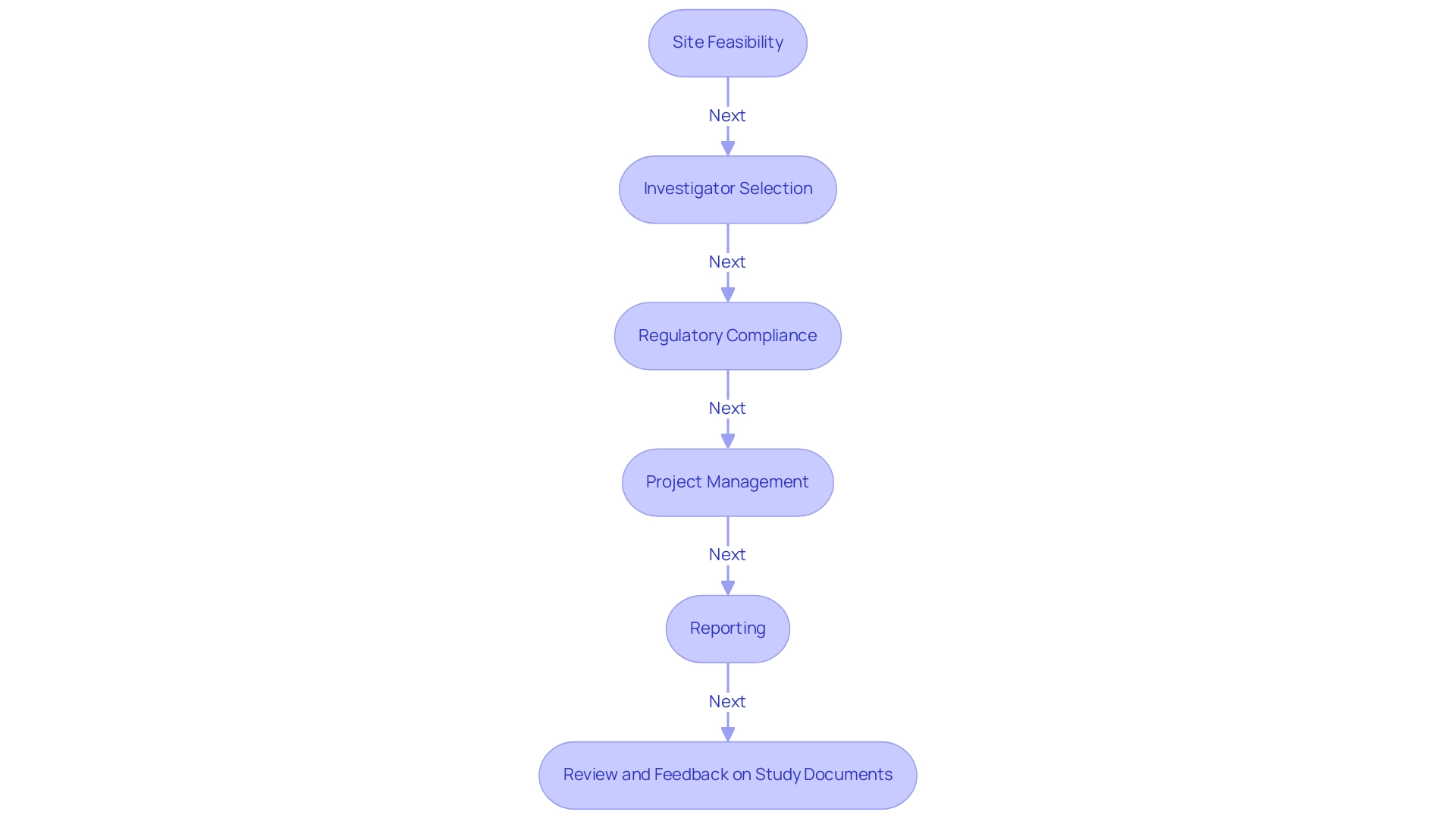
Clinical Research Organizations (CROs) play a crucial role in shaping the future of medtech trials in Latin America, particularly in Colombia, which provides competitive advantages such as cost efficiency, regulatory speed, high-quality healthcare, and strong patient recruitment capabilities. Companies like bioaccess® exemplify the tailored support that CROs provide, navigating the complexities of studies with a comprehensive process that includes:
- Site feasibility
- Investigator selection
- Regulatory compliance
- Project management
- Reporting
- Review and feedback on study documents to comply with country requirements
Additionally, they assist with import permits and nationalization of investigational devices.
This strategic collaboration allows researchers to concentrate on their primary objectives while enhancing trial efficiency. For example, Medpace's dedication to creating 1,500 positions and investing $150 million over the next six years indicates a strong investment in research capabilities, which could significantly benefit local Medtech initiatives. Moreover, Proclinical's search for an External Manufacturing Operations Lead in Slough, England, demonstrates the increasing demand for skilled professionals in the CRO sector, a trend that may affect hiring strategies in the southern region as the market grows.
Additionally, Precision for Medicine's acquisition of Baseline Controls is poised to accelerate biomanufacturing processes, particularly in oncology and gene therapy, leading to faster drug market entry—a clear illustration of how CROs can enhance operational efficiency in these critical areas. As Michelle Keefe, CEO of Syneos Health, emphasized, organizations are progressively embracing data-driven methods to enhance research outcomes, a trend that is especially pertinent to CROs functioning in Latin America. The landscape of CROs in the region is poised to grow substantially in 2024, which will significantly impact research efficiency, making their collaboration essential for the future of medtech trials in Latin America.
To investigate how we can assist with your research requirements, SCHEDULE A MEETING with us today.
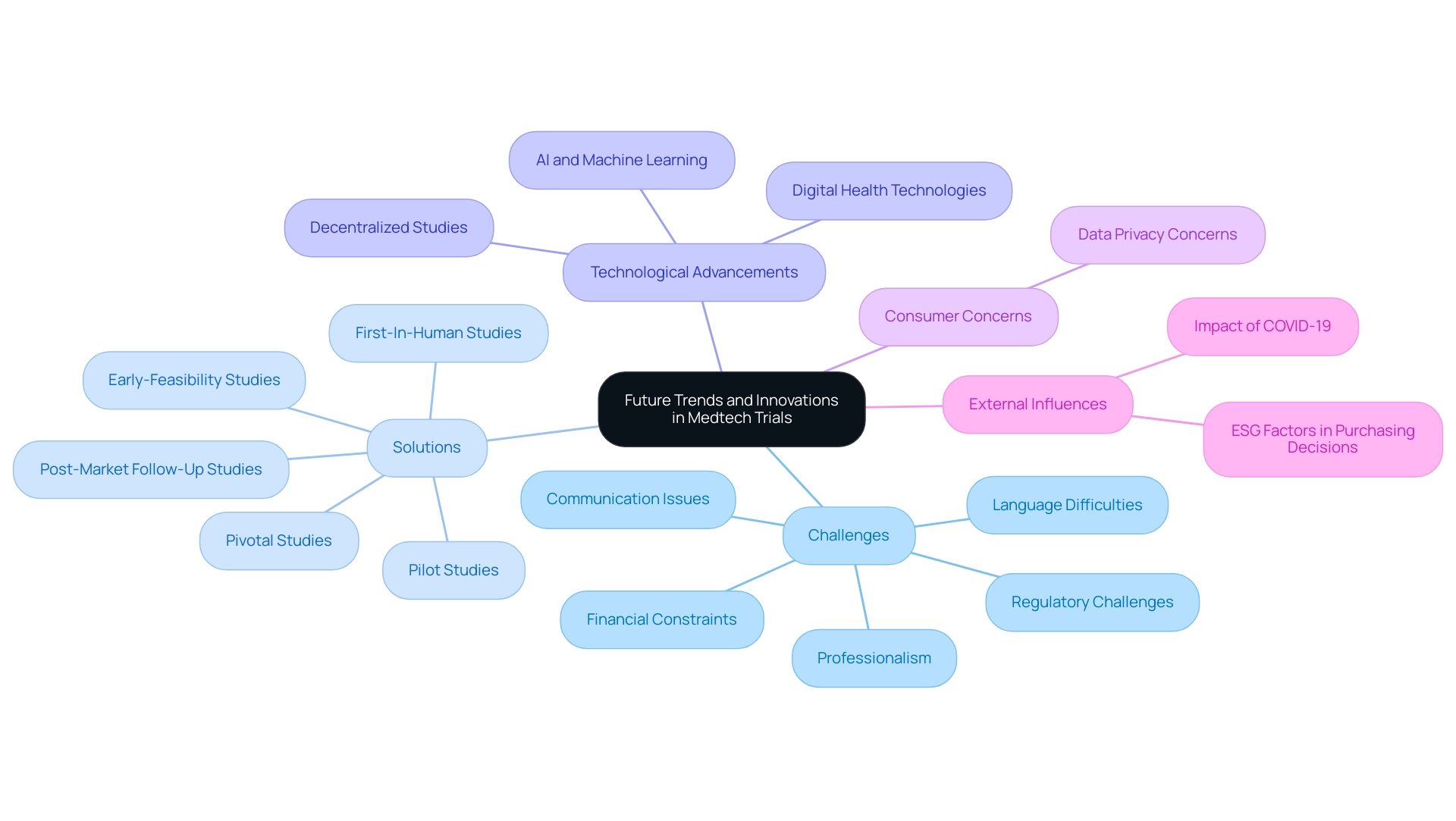
Future Trends and Innovations in Medtech Trials
The landscape of Medtech studies in Latin America is on the brink of significant transformation, greatly influenced by the future of medtech trials in Latin America, rapid technological advancements, and innovative methodologies. Medtech firms encounter diverse obstacles, such as:
- Regulatory challenges
- Financial constraints
- Professionalism
- Language difficulties
- Communication issues
These challenges are being tackled by bioaccess® through its extensive research management services, including:
- Early-Feasibility Studies
- First-In-Human Studies
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies
As digital health technologies, including telemedicine and mobile health applications, become increasingly integrated into study designs, decentralized studies are emerging, enhancing patient engagement and streamlining data collection processes.
Collaboration between Greenlight Guru and bioaccess™ exemplifies the effort to accelerate Medtech innovations and clinical studies in the region, highlighted by PAVmed's first-in-human study in Colombia. Additionally, a substantial 70% of healthcare consumers express concerns about data privacy, emphasizing the need for secure digital solutions in research frameworks. Concurrently, with 70% of MedTech customers integrating environmental, social, and governance (ESG) factors into their purchasing decisions, it becomes paramount for the industry to adapt.
The rise of artificial intelligence and machine learning is revolutionizing data analysis, leading to more efficient patient recruitment and ongoing monitoring. However, challenges such as:
- Cybersecurity threats
- Evolving policies
- Fragmentation of resources
require researchers to remain vigilant and adaptable. The impact of COVID-19 has further emphasized the necessity for flexibility and innovation in study designs.
As we approach 2024, leveraging these technologies will be critical in shaping the future of medtech trials in Latin America, ensuring they are optimized for efficacy, compliance, and positive economic impacts such as job creation and healthcare improvement.
Conclusion
The Medtech trial landscape in Latin America is poised for remarkable growth, driven by a unique combination of regulatory advantages, a diverse patient population, and strategic investments. Countries like Brazil, Mexico, and Argentina are emerging as key players, while Chile is innovating with regulatory frameworks that enhance its position as a hub for clinical trials. Success stories, such as ReGelTec's Early Feasibility Study and Avantec Vascular's pioneering clinical studies, illustrate the region's potential for advancing innovative therapies and improving healthcare outcomes.
Navigating the regulatory complexities is crucial for researchers aiming to capitalize on the opportunities in Latin America. With countries like Colombia offering competitive advantages and incentives, such as R&D tax credits, it is clear that engaging local regulatory authorities and utilizing the expertise of Clinical Research Organizations (CROs) can significantly streamline the trial process. The collaborative spirit between US and Latin American firms further enhances the research environment, fostering innovation and driving advancements in medical technology.
Looking ahead, the integration of digital health technologies and decentralized trial designs will redefine the Medtech landscape in the region. As challenges such as regulatory hurdles and data privacy concerns persist, the focus on innovative solutions and strategic partnerships will be vital for success. The ongoing evolution of the Medtech sector in Latin America not only promises to enhance clinical trial efficiency but also offers the potential for improved healthcare outcomes, ultimately positioning the region as a formidable player in the global Medtech arena. Embracing this transformative journey will be essential for stakeholders aiming to make a meaningful impact in the healthcare landscape.
Frequently Asked Questions
What is the current landscape of medtech trials in Latin America?
The medtech testing environment in Latin America is rapidly evolving, with Brazil, Mexico, and Argentina emerging as key contributors. Chile stands out due to its strategic investments and regulatory advancements, making it a significant influencer in the future of medtech trials.
What factors contribute to the growth of medtech trials in Latin America?
Regulatory benefits, a large patient base, and significant investments in healthcare innovation are driving the expansion of medtech trials in the region.
How does Chile differentiate itself in medtech trials?
Chile combines regulatory speed, ethical oversight, and a robust healthcare system, positioning itself as a leader in shaping the future of medtech trials in Latin America.
Can you provide examples of recent medtech studies conducted in Latin America?
Yes, recent studies include: ReGelTec's Early Feasibility Study on HYDRAFIL™ for chronic low back pain, which treated eleven patients in Barranquilla; Avantec Vascular's first-in-human research of a vascular device, supported by bioaccess™; and a partnership between GlobalCare Clinical Studies and bioaccess™, which improved recruitment time and retention rates for clinical studies in Colombia.
What are the main segments of the medical technology market in Latin America?
The medical technology market consists of two key segments: In Vitro Diagnostics (IVD) and Medical Devices.
What challenges do researchers face in conducting medtech trials in Latin America?
Researchers must navigate diverse regulatory frameworks, cultural nuances, and resource limitations that can affect the effectiveness of clinical studies.
What is the significance of the Colombian R&D tax credit for medtech companies?
The Colombian R&D tax credit allows small and midsize businesses to claim a 50% tax credit on R&D and innovation initiatives, providing a financial incentive for conducting studies in the country.
What steps are involved in securing research study approval in Colombia?
The approval process includes several critical steps: 1. IRB/EC approval 2. INVIMA approval 3. Obtaining a MinCIT import permit.
How can regulatory consultants assist in the approval process for medtech trials?
Regulatory consultants, such as bioaccess®, can provide support throughout the research process, ensuring compliance with regulations and facilitating a more expedited approval process.
Why is Colombia becoming a desirable location for clinical trials?
Colombia is recognized for its competitive advantages, including an ambitious science, technology, and innovation plan, making it an increasingly attractive destination for conducting clinical trials in the region.

