Introduction
The Premarket Approval (PMA) process stands as a critical regulatory framework established by the U.S. Food and Drug Administration (FDA) to ensure the safety and efficacy of high-risk medical devices. Unlike the more streamlined 510(k) pathway, PMA demands rigorous clinical data and comprehensive evaluations, reflecting the heightened concerns surrounding patient health.
As the landscape of medical technology continues to evolve, understanding the nuances of the PMA process becomes paramount for developers aiming to navigate the complexities of regulatory compliance.
This article delves into the intricacies of the PMA application process, compares it with other regulatory pathways, and highlights the indispensable role of clinical trials and post-approval compliance in safeguarding patient well-being.
Through a thorough examination of these elements, stakeholders can better strategize their approach to market entry and ensure adherence to the highest standards of device safety.
Introduction to Premarket Approval (PMA) in Medical Devices
The Premarket Approval (PMA) procedure represents a comprehensive regulatory pathway created by the U.S. Food and Drug Administration (FDA) designed for medical instruments that pose significant risks to patient safety. Unlike the 510(k) pathway, which allows items to receive approval by showing substantial equivalence to existing products, the PMA pathway requires the submission of extensive clinical data that verifies both the safety and effectiveness of the product in question. This rigorous requirement is designed to ensure that only those products adhering to the highest safety standards are permitted to enter the market, thereby safeguarding patient health and well-being.
For developers, a thorough understanding of the PMA pathway is essential, as it not only delineates the level of evidence necessary for approval but also informs the strategic framework for device development. In the context of Latin America, where Medtech firms encounter distinct obstacles such as compliance challenges, language barriers, and resource fragmentation, collaboration with specialists like bioaccess® can significantly improve the clinical study process. Bioaccess® offers a range of clinical trial management services, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Post-Market Clinical Follow-Up Studies (PMCF)
These services are critical in navigating these challenges.
As the FDA continues to navigate an evolving regulatory landscape, the average panel track wait times have increased slightly to 289.62 days in 2024. This rise, representing a 1.3% increase, could impact approval timelines and market strategies, making it crucial for stakeholders to adapt accordingly. Furthermore, as noted by Murad Alam, MD, respondents have expressed a desire for improved study designs and more relevant clinical data, emphasizing the need for panelists to be involved in study design and device label development.
Furthermore, 26.3% of respondents expressed worries that executive sessions might extend the decision-making time, emphasizing a vital viewpoint on the PMA pathway that stakeholders must consider. This underscores the urgent need for a solution-driven approach to bridge the gap between innovation and execution in Latin America.
Navigating the PMA Application Process: Key Steps and Requirements
The pma pathway application process is intricate and requires careful navigation through several key steps:
- Pre-Submission Meeting: Engaging with the FDA early on is paramount. This step enables developers to discuss their equipment, clarify regulatory expectations, and gather crucial feedback that can inform subsequent steps.
- Investigational Device Exemption (IDE): Should clinical studies be necessary, submitting an IDE is essential. This exemption permits the investigation of the device in human subjects, providing a framework for data collection.
- Clinical Studies: Conducting comprehensive studies is vital for gathering robust data on safety and efficacy. Well-structured studies can significantly affect the FDA's evaluation.
Here, utilizing bioaccess®'s expertise in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), and Pivotal Studies can enhance the study's efficacy and compliance with local regulations. Additionally, bioaccess® provides essential services such as site selection, compliance reviews, and thorough project management, ensuring that all trial setup and approval processes are meticulously handled.
- The pma pathway is an important focus in our research.
- PMA Submission: The compilation of the PMA application must be meticulous, including all clinical data, manufacturing details, and labeling information necessary for the pma pathway. This comprehensive submission is critical for the FDA's evaluation.
- FDA Review: The FDA's review period can extend over several months, during which the agency may request additional information or clarification, emphasizing the importance of responsiveness. According to compliance insights, failing to respond within 180 days may lead the FDA to consider the PMA withdrawn voluntarily, underscoring the need for timely communication.
- Approval: Upon meeting all FDA standards, the PMA is granted, allowing the product to enter the market. Comprehending these steps, along with the obstacles encountered by medical equipment startups—such as compliance challenges and financial limitations—within the pma pathway is essential for securing a seamless approval. Specialists, such as Katherine Ruiz, a noted expert in compliance matters for medical equipment in Colombia, emphasize that strategic planning and cooperation with compliance professionals can significantly improve submission efficiency and results in the medical technology field.
Furthermore, the importance of post-market clinical follow-up studies (PMCF) cannot be overstated, as they provide ongoing safety and efficacy data that is essential for maintaining compliance with FDA standards. This is backed by the case study titled 'Strategic Planning for PMA Approval', which demonstrates how stakeholders must assess the PMA process carefully, taking into account factors such as feasibility studies and compliance reviews to create informed oversight strategies.
Comparing PMA with Other Regulatory Pathways: 510(k) and De Novo
The medical equipment approval landscape is governed by three distinct pathways: PMA, 510(k), and De Novo, each tailored to different categories and regulatory needs. The Premarket Approval (PMA) pathway is specifically designed for high-risk products that require robust clinical data to ensure safety and efficacy. This procedure is thorough, showcasing the essential nature of the instruments involved.
In fact, among the authorized Breakthrough Instruments, there are currently 124 CDRH items and 4 CBER items, highlighting the significant volume of products navigating the PMA pathway. Conversely, the 510(k) process allows for a more streamlined approval route for products that are substantially equivalent to existing, legally marketed counterparts. This mechanism can significantly expedite market entry, although it is essential to note that products cleared under this pathway cannot be marketed as 'FDA-approved.'
As analyst Iseult McMahon notes, 'We think that this is a directional positive for the broader medical technology space as it provides both improving certainty to companies (and investors) as well as reduces cash burn during the interim period when a company is awaiting a decision.' Lastly, the De Novo pathway caters to novel devices classified as low to moderate risk, which lack a predicate device. This approach offers a risk-based classification system that can facilitate quicker access to market while ensuring appropriate oversight.
A practical example of this is the Q-LINEA AB ASTAR BC G-KIT AND ASTAR INSTRUMENT, which was authorized on April 26, 2024, under submission number K221688, demonstrating the successful navigation of the approval process. At bioaccess®, we offer comprehensive clinical trial management services including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
Our expertise guarantees the success of your clinical studies in Latin America by aligning our services with the specific compliance pathways, including the PMA pathway, 510(k), and De Novo.
Choosing the suitable compliance route is essential, as it directly affects the timeline, related expenses, and the amount of information needed to launch a product. Our specialist, Katherine Ruiz, focuses on regulatory matters for medical equipment and in vitro diagnostics in Colombia, ensuring you maneuver through the intricacies of these procedures efficiently.
The Role of Clinical Trials in the PMA Process
Clinical studies are essential to the PMA pathway, as they provide the necessary evidence to support claims of safety and effectiveness for medical instruments. Developers must meticulously design clinical trials that not only comply with FDA regulations but also accurately reflect the intended use of the device. Critical considerations in this process include:
- The selection of appropriate endpoints
- Ensuring an adequate sample size
- Implementing rigorous data collection and analysis methodologies
Furthermore, anticipating potential challenges, such as patient recruitment and regulatory scrutiny, is essential. Recent statistics indicate that over 80% of Original PMAs filed in 2021 had major deficiencies, highlighting the pressing need for well-organized studies. A notable example is ReGelTec's Early Feasibility Study in Barranquilla, Colombia, where eleven patients with degenerative disc disease were successfully treated with HYDRAFIL™, a patented hydrogel injected into the nucleus of a degenerated disc via a 17-gauge needle.
This demonstrates effective study design, as all eleven patients were treated successfully. Establishing a robust testing protocol and engaging with regulatory bodies early can facilitate smoother navigation through the review process. Bioaccess® enhances clinical study management services, achieving over 50% reduction in recruitment time and 95% retention rates, further emphasizing the value of expertise in Early-Feasibility, First-In-Human, and other pivotal studies.
Additionally, GlobalCare Clinical Studies partners with bioaccess® to bolster clinical study ambulatory services. As Katrina Rogers emphasizes,
If prompt evaluation of the submission is vital to your business, you should consider writing your Senator to express your support for this program.
Ultimately, well-conducted clinical studies can significantly enhance the probability of successful approval through the PMA pathway, emphasizing their significance in the regulatory framework for medical equipment.
Post-Approval Compliance: Ensuring Ongoing Safety and Effectiveness
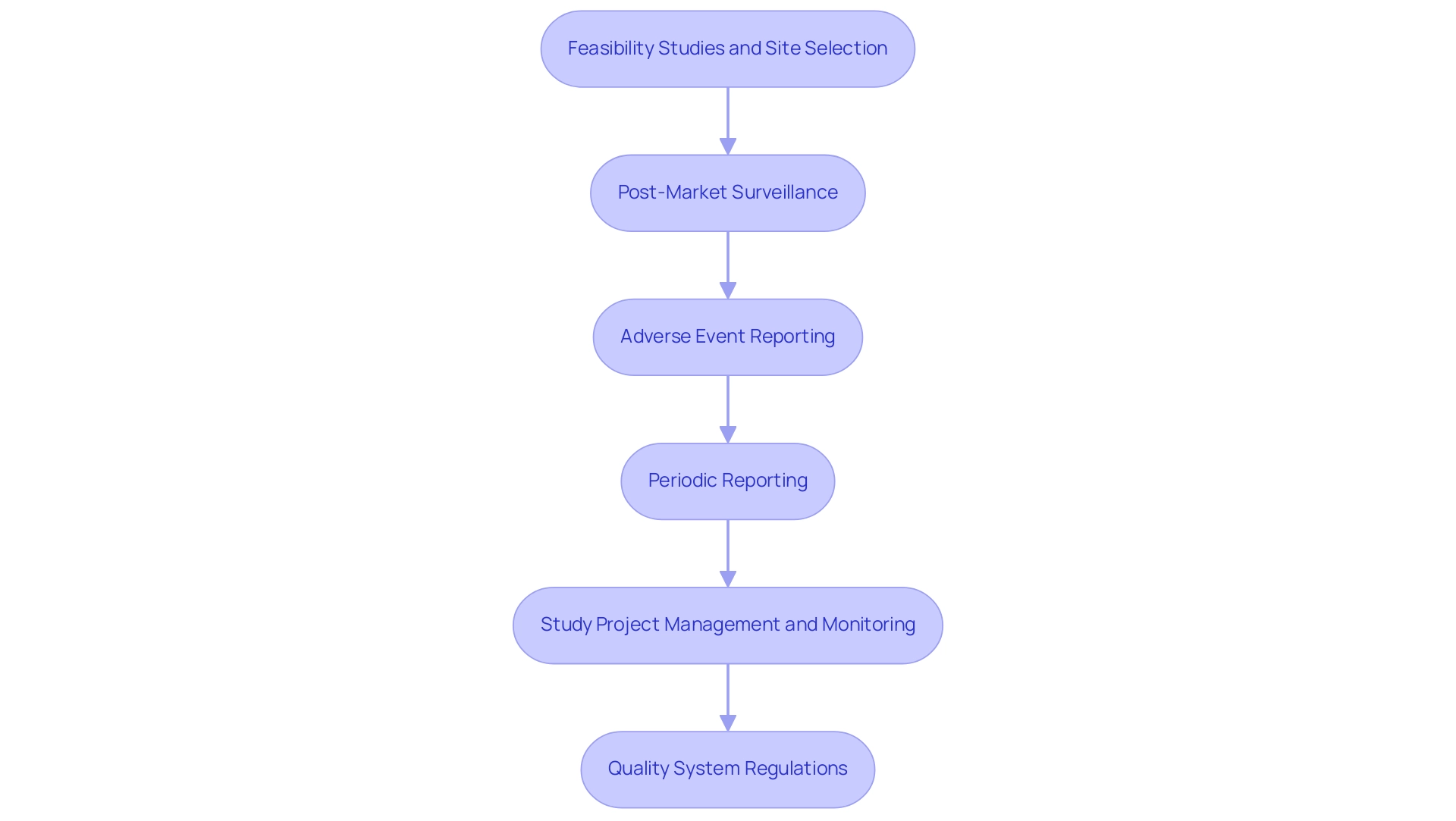
After securing PMA approval, medical device developers must commit to various ongoing compliance requirements to ensure the sustained safety and efficacy of their products. Our extensive clinical study management services are vital in this procedure, which includes:
-
Feasibility Studies and Site Selection: Identifying appropriate research sites and principal investigators (PIs) is vital for the success of clinical trials.
Our team conducts thorough feasibility assessments to ensure that selected sites meet the necessary criteria for effective study execution.
-
Post-Market Surveillance (PMS): This critical process involves continuously monitoring the product's real-world performance to detect any potential safety concerns.
A proactive PMS enhances safety, customer satisfaction, and fosters ongoing business improvement. Regular staff training on PMS procedures, as highlighted in the case study titled 'Training and Continuous Improvement in PMS,' is essential for refining the PMS system and improving outcomes.
-
Adverse Event Reporting: Developers are required to promptly report any adverse events or failures to the FDA, thereby facilitating timely action and risk mitigation.
-
Periodic Reporting: Regular submission of performance reports to the FDA is essential, detailing the device’s functionality and any modifications in manufacturing processes.
-
Study Project Management and Monitoring: Effective project management ensures that all aspects of the clinical trial are executed according to plan, with continuous monitoring to address any issues that arise.
This involves monitoring study status and inventory, ensuring that all components are in accordance with legal requirements.
-
Quality System Regulations (QSR): Complying with QSR guarantees that the product is produced according to its approved specifications, maintaining quality and reliability.
The FDA emphasizes its intent to review the Post-Approval Study (PAS) protocol interactively with the sponsor during the PMA review, reinforcing the significance of these compliance measures.
Katherine Ruiz, an expert in Regulatory Affairs for medical instruments and in vitro diagnostics in Colombia, emphasizes the significance of these ongoing compliance requirements. Additionally, the Event Location Code for the RADIOLOGY DEPARTMENT (511) serves as a reminder of the specific environments where ongoing compliance is critical.
By thoroughly understanding and implementing these post-approval compliance requirements within the PMA pathway, developers contribute significantly to the safety and effectiveness of their devices, ultimately benefiting users and enhancing market confidence.
Conclusion
Understanding the intricacies of the Premarket Approval (PMA) process is essential for developers of high-risk medical devices. This comprehensive regulatory pathway, governed by the FDA, demands rigorous clinical data and thorough evaluations to ensure that only the safest and most effective devices reach the market. The article highlights the critical steps involved, including:
- Pre-submission meetings
- Clinical trials
- The meticulous PMA submission process
This emphasizes the necessity of structured trials and timely communication with regulatory bodies.
Furthermore, the comparison of PMA with other regulatory pathways, such as the 510(k) and De Novo processes, underscores the unique challenges faced by developers in navigating the regulatory landscape. The importance of clinical trials in substantiating device safety and efficacy cannot be overstated, as evidenced by the significant number of deficiencies noted in past submissions. Engaging expert services, such as those offered by bioaccess®, can streamline this process and enhance compliance with local regulations.
Post-approval compliance also plays a vital role in maintaining the safety and effectiveness of medical devices. Ongoing monitoring, adverse event reporting, and adherence to quality system regulations are crucial for ensuring that devices continue to meet regulatory standards after entering the market. By fully grasping these elements of the PMA process, stakeholders can better strategize their market entry and contribute to the overall enhancement of patient safety and device reliability. The commitment to rigorous compliance and proactive management ultimately fosters greater confidence in the medical technology sector, paving the way for innovation and improved health outcomes.
Frequently Asked Questions
What is the Premarket Approval (PMA) procedure?
The PMA procedure is a regulatory pathway established by the U.S. Food and Drug Administration (FDA) for medical instruments that pose significant risks to patient safety. It requires extensive clinical data to verify both the safety and effectiveness of the product.
How does the PMA pathway differ from the 510(k) pathway?
Unlike the 510(k) pathway, which allows approval by demonstrating substantial equivalence to existing products, the PMA pathway requires comprehensive clinical data to ensure the highest safety standards for new medical devices.
Why is understanding the PMA pathway important for developers?
A thorough understanding of the PMA pathway is crucial for developers as it outlines the necessary evidence for approval and informs the strategic framework for device development.
What challenges do Medtech firms face in Latin America regarding the PMA pathway?
Medtech firms in Latin America encounter compliance challenges, language barriers, and resource fragmentation, making collaboration with specialists like bioaccess® beneficial for improving the clinical study process.
What clinical trial management services does bioaccess® offer?
Bioaccess® offers services including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), and Post-Market Clinical Follow-Up Studies (PMCF).
What is the average panel track wait time for PMA in 2024?
The average panel track wait time for PMA in 2024 is 289.62 days, reflecting a 1.3% increase from previous years.
What concerns have been raised by respondents regarding the PMA pathway?
Respondents have expressed a desire for improved study designs and more relevant clinical data, as well as concerns that executive sessions might extend decision-making times.
What are the key steps in the PMA application process?
The key steps include: 1. Pre-Submission Meeting with the FDA 2. Submission of Investigational Device Exemption (IDE) 3. Conducting Clinical Studies 4. Compiling the PMA Submission 5. FDA Review 6. Approval.
What happens during the FDA review period for a PMA application?
The FDA review period can extend for several months, during which the agency may request additional information or clarification. Failure to respond within 180 days may lead to the PMA being considered withdrawn voluntarily.
Why are post-market clinical follow-up studies (PMCF) important?
PMCF studies provide ongoing safety and efficacy data necessary for maintaining compliance with FDA standards after a product has entered the market.




