Introduction
In the complex landscape of clinical trials, the definition and selection of endpoints are paramount to evaluating the effectiveness and safety of new treatments. These specific outcomes not only guide trial design but also play a critical role in regulatory decisions, impacting the approval of innovative therapies.
As the field evolves, particularly in regions like Latin America, Medtech companies encounter unique challenges that can influence the success of clinical trials. From understanding primary and secondary endpoints to navigating the intricacies of surrogate markers, researchers must adopt a meticulous approach to endpoint selection.
This article delves into the significance of clinical trial endpoints, exploring their types, the importance of accurate measurement, and the challenges faced in their implementation, ultimately underscoring their role in shaping the future of medical research and patient care.
Defining Clinical Trial Endpoints: An Overview
Clinical study objectives are specified as particular events or results that a research aims to assess, which include the types of endpoints in clinical trials, ultimately determining the impacts of a treatment or intervention. These targets can encompass a wide range of metrics, which are considered types of endpoints in clinical trials, including:
- Survival rates
- Disease progression
- Quality of life indicators
A comprehensive grasp of the types of endpoints in clinical trials is crucial, as they not only guide the study's structure but also significantly impact regulatory choices.
In the context of Latin America, Medtech companies face unique challenges, such as:
- Regulatory hurdles
- Professionalism issues
- Communication barriers
- Fragmentation of resources
These challenges can impact the effectiveness of clinical trials. For instance, in the analysis of patients with metastatic melanoma, tracking progression-free survival is one of the types of endpoints in clinical trials that is particularly critical for individuals aged 12 years and older, as it informs treatment effectiveness. Similarly, patients with type 1 diabetes mellitus are evaluated through serum hemoglobin A1C levels, which serve as a traditional approval criterion for glucose-lowering therapies and are considered one of the types of endpoints in clinical trials.
Partnership between firms such as Greenlight Guru and bioaccess™ is essential to speed up Medtech innovations in the area, underscored by PAVmed's first-in-human research in Colombia. Moreover, the successful implementation of medical studies can result in considerable local economic effects, such as job generation and healthcare advancements. For instance, research has demonstrated that medical studies can enhance local employment rates by generating specialized positions in research and healthcare.
In the current landscape, sponsors must carefully evaluate whether increases in complexity are necessary, balancing cost, speed, and intricacy. As Ken Getz, an expert in the field, emphasizes, 'Collective investment into leveraging popular culture and mass media as an educational medium is urgently needed.' This declaration highlights the need for clear communication about the significance of study objectives.
Moreover, media coverage, like that offered by Clinical Leader, plays an essential role in increasing awareness about research studies in Latin America and can assist in closing the knowledge gap. Ultimately, these points serve as standards for assessing the effectiveness and safety of new medications or treatment methods, which are categorized as types of endpoints in clinical trials, influencing the environment of research and regulatory results.
Exploring Different Types of Endpoints in Clinical Trials
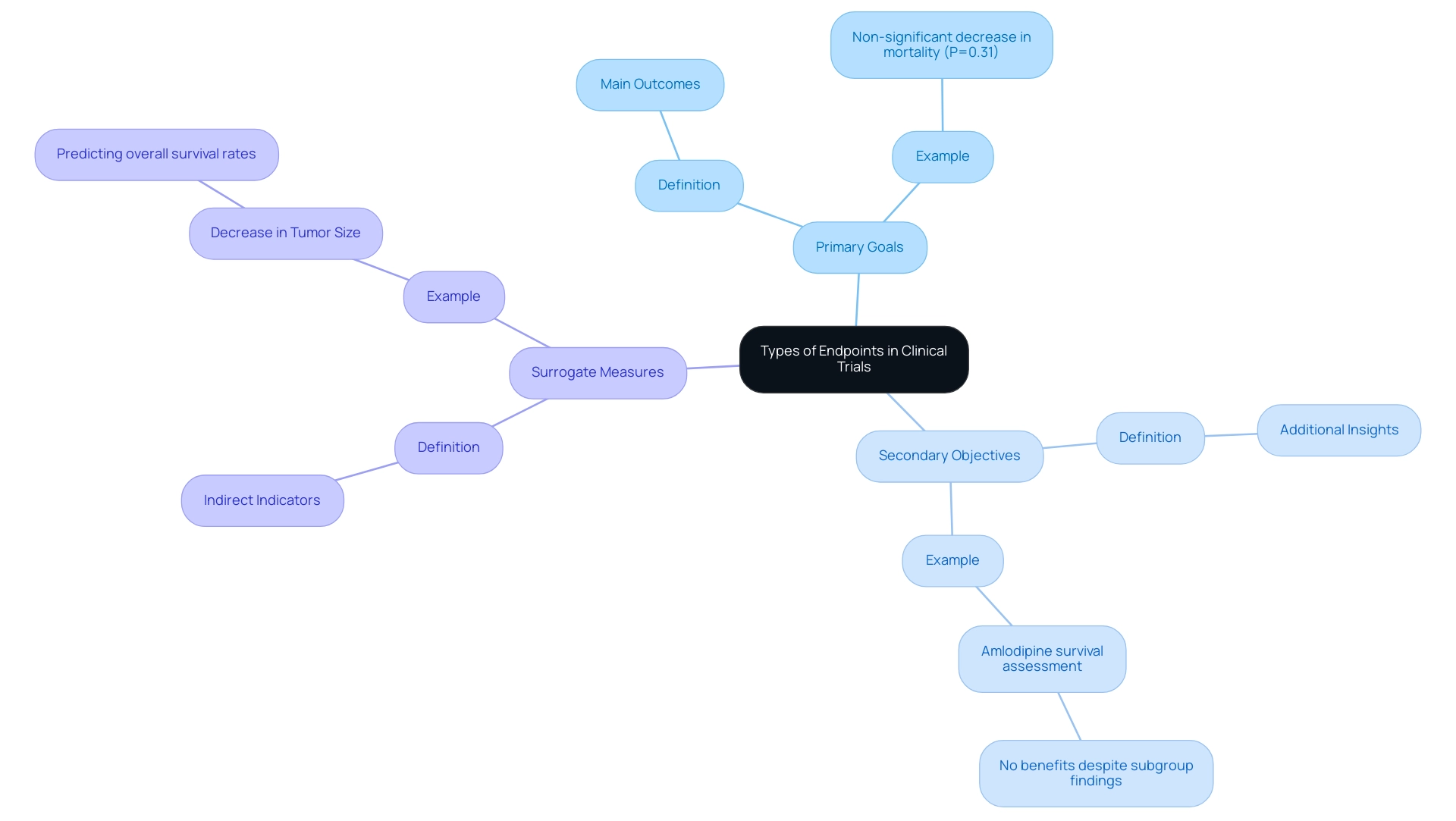
In the domain of clinical studies, objectives are critically classified into types of endpoints in clinical trials, including primary, secondary, and surrogate goals.
- Primary goals signify the main outcomes that a trial is specifically designed to measure, providing the core evidence necessary to evaluate a treatment's efficacy.
- Secondary objectives provide additional insights that can enhance the understanding of a treatment’s effects.
Surrogate measures, on the other hand, serve as indirect indicators that aim to predict clinical outcomes. A relevant example can be found in cancer studies, where a decrease in tumor size is frequently used as a substitute measure to infer possible enhancements in overall survival rates.
Grasping these differences is crucial for researchers as they create effective investigations and interpret the types of endpoints in clinical trials with precision. For example, the main outcome measure in a recent study demonstrated a non-significant decrease in mortality from any cause or hospitalization for major cardiovascular incidents (P=0.31), emphasizing the significance of outcome selection in clinical research. Furthermore, the second potential randomized amlodipine survival assessment revealed no advantages for amlodipine despite notable subgroup findings, acting as a cautionary illustration of outcome interpretation and its implications.
Moreover, a case study titled 'Improved Survival for Children and Young Adults with T-Lineage Acute Lymphoblastic Leukemia' analyzed outcomes from the Children’s Oncology Group AALL0434 study, showing improved survival rates due to methotrexate randomization. This highlights the real-world significance of comprehending primary and secondary objectives. As pointed out by Dr. Mei-Jie Zhang, a prominent authority in medical research, the capability to effectively assess outcomes of research studies, particularly randomized controlled studies (RCT), is an essential skill for every physician.
This highlights the significance of a thorough understanding of the types of endpoints in clinical trials to improve the reliability of study results and their implications in medical practice.
The Importance of Endpoint Selection in Clinical Trials
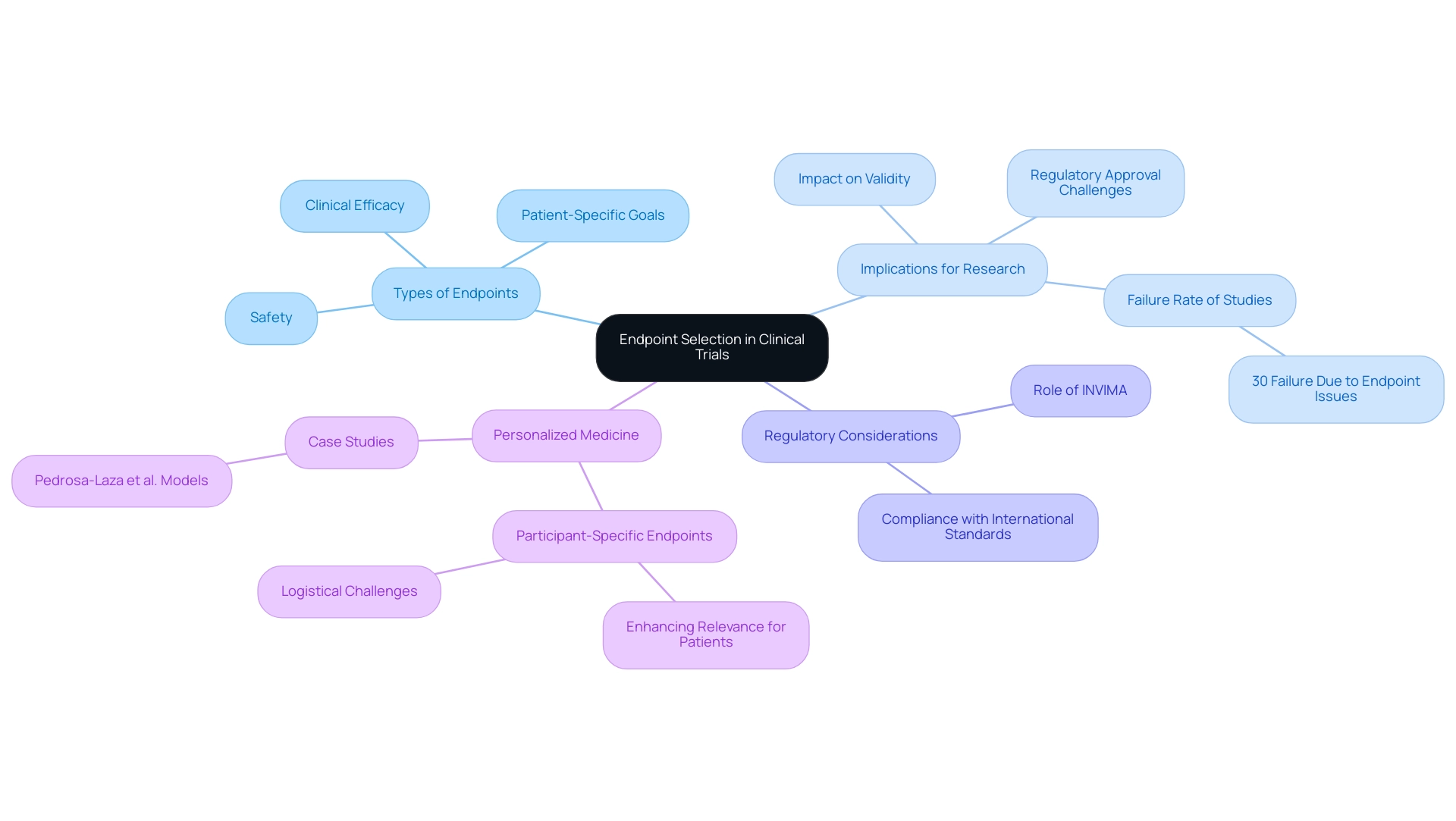
The choice of suitable targets is a crucial element of clinical study design, directly affecting the validity and applicability of the research's findings. The types of endpoints in clinical trials must not only align with the study's objectives but also provide meaningful insights into treatment efficacy and safety. A failure to choose appropriate targets can lead to inconclusive results or misinterpretation of data, jeopardizing the chances of regulatory approval.
Recent discussions highlight that approximately 30% of medical studies fail due to issues with the types of endpoints in clinical trials, underscoring the need for meticulous planning. As Ken Getz notes,
Collective investment into leveraging popular culture and mass media as an educational medium is urgently needed,
reflecting the current need for enhanced understanding in this area. Additionally, Clinical Leader has released multiple articles discussing media coverage of clinical studies in Latin America and Colombia, which can assist in spreading essential information about outcome selection and its implications.
Additionally, the development of participant-specific goals represents a promising approach to personalizing medicine by identifying various types of endpoints in clinical trials. These points, while improving the significance of discoveries for individual patients, introduce complexities that can restrict the applicability of study results. For instance, Pedrosa-Laza et al.
created treatment models to predict time until hospitalization due to COVID-19, illustrating the influence of outcome selection on patient results. Moreover, INVIMA plays a crucial role as Colombia's regulatory body, overseeing medical device classifications and ensuring compliance with international standards as a Level 4 health authority recognized by PAHO/WHO. This position is essential in influencing the regulatory framework for research studies in Colombia, as it directly affects the approval procedure and the selection of types of endpoints in clinical trials.
The extra financing obtained from the Yale Clinical and Translational Science Award (UL1 TR001863) highlights the significance of thorough outcome selection in research studies. Consequently, it is crucial for researchers to carefully examine the practical significance, statistical soundness, and feasibility of assessing each objective during the study planning phase to enhance research results.
Biomarkers and Surrogate Endpoints: Their Role in Clinical Trials
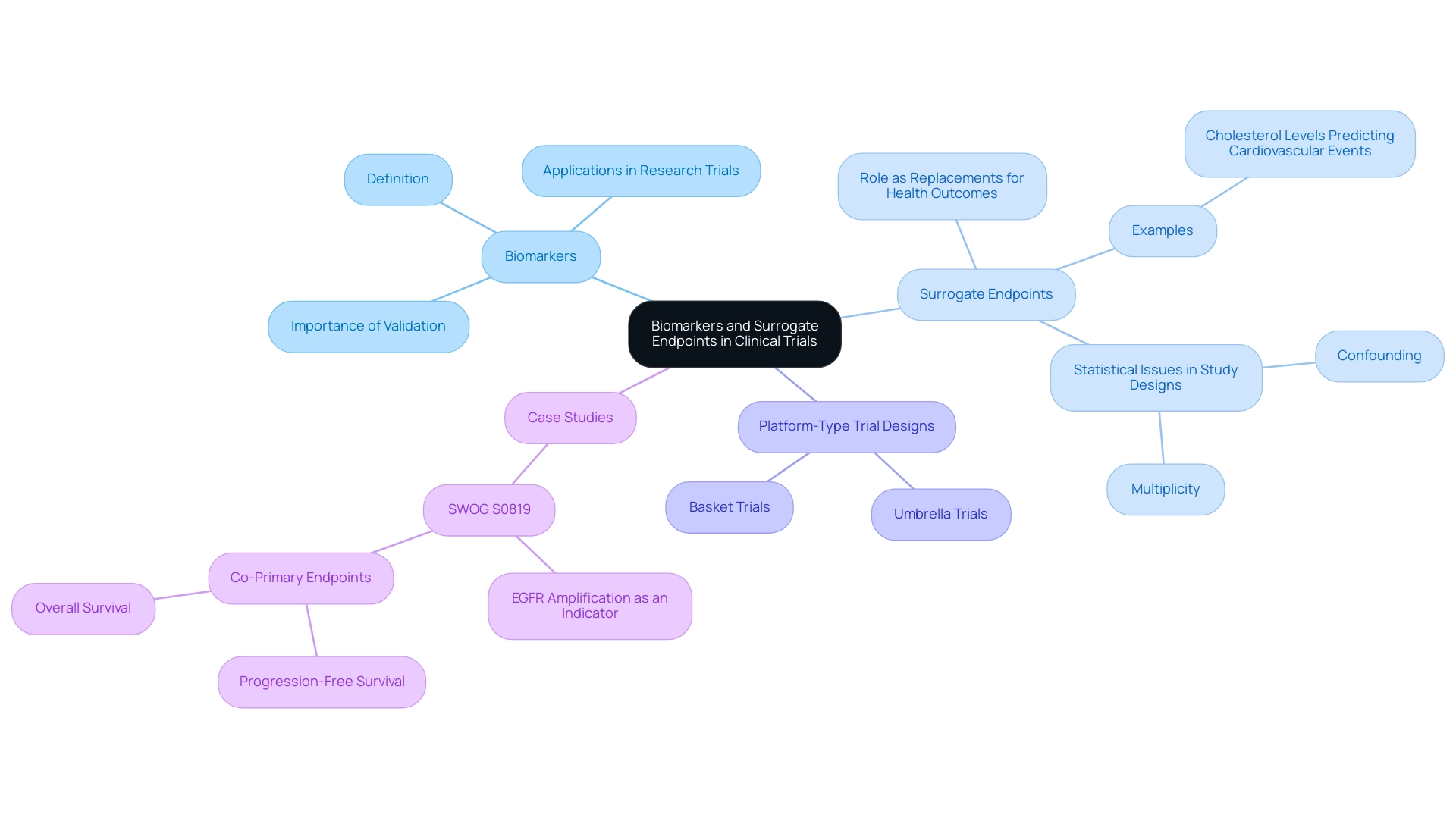
Biomarkers function as essential biological indicators that inform researchers about the effects of treatments in research trials. In contrast, surrogate markers are a particular type of biomarkers that function as replacements for direct health outcomes, thus offering a convenient measure of treatment effectiveness. For example, a reduction in cholesterol levels may be utilized as a substitute measure to predict the likelihood of subsequent cardiovascular events.
The strategic application of biomarkers and substitute measures can significantly simplify the research process, enabling scientists to achieve quicker results and potentially accelerating the approval of new treatments. However, it is essential to emphasize that the implementation of these indicators requires thorough validation. Such validation is crucial to ensure that biomarkers and surrogate endpoints reliably predict clinical outcomes.
A systematic review published in BMC Cancer in 2019 highlighted statistical issues related to study designs incorporating biomarkers, including confounding and multiplicity, and provided insights into the effectiveness of various study designs. Notably, platform-type trial designs, such as umbrella and basket trials, facilitate biomarker validation by allowing for the simultaneous testing of multiple treatments across different patient populations. In this context, the insights from experts, such as Isao Yoshimura from Tokyo University of Science, highlight the collaborative nature of biomarker research:
We are grateful to Nan M. Laird at the Department of Biostatistics, Harvard School of Public Health, and Isao Yoshimura at Tokyo University of Science for their valuable advice and suggestions.
This collaborative approach is vital for fostering advancements in the field and ensuring the robust validation of substitute measures. For example, the case analysis of SWOG S0819 investigated the effectiveness of EGFR amplification as an indicator for treatment advantages in patients receiving EGFR antibodies alongside chemotherapy, highlighting how alternative measures can influence treatment choices in medical practice.
Challenges in Measuring Clinical Trial Endpoints
Measuring types of endpoints in clinical trials presents numerous challenges that can significantly influence results. Key issues include:
- Variability in data collection methods
- Participant adherence to study protocols
- Subjective interpretation of results
Many trials utilize standardized criteria, such as those defined by the Response Evaluation Criteria in Solid Tumors guidelines, to measure treatment responses.
However, the controversy surrounding progression-free survival as a meaningful measure of clinical benefit remains a critical topic, as it does not always correlate with overall survival. This debate highlights the need for careful consideration in the selection of types of endpoints in clinical trials, as relying solely on progression-free survival may not adequately reflect patient outcomes. Inconsistent data collection practices can introduce bias, and low participant retention rates may compromise the study's statistical power. As highlighted by R Daniel Meyer, tackling these statistical problems is especially vital in the context of experiments conducted during extraordinary situations such as the COVID-19 pandemic.
To effectively mitigate these challenges, researchers should:
- Establish clear protocols
- Thoroughly train staff
- Implement robust data management systems
Ensuring consistent data collection is vital not only for the reliability and validity of trial results but also for influencing regulatory decisions and safeguarding patient safety.
For instance, a randomized trial examining T-lineage acute lymphoblastic leukemia demonstrated improved survival rates through a targeted methotrexate randomization strategy. This case study underscores the potential impact of rigorous measurement and data collection methods on the types of endpoints in clinical trials, reinforcing the importance of selecting appropriate types of endpoints in clinical trials that truly reflect patient benefit.
Conclusion
The significance of clinical trial endpoints cannot be overstated, as they serve as the foundation upon which the efficacy and safety of new treatments are assessed. A comprehensive understanding of primary, secondary, and surrogate endpoints is essential for researchers navigating the complexities of trial design and execution. By meticulously selecting appropriate endpoints, researchers can enhance the validity of study findings and ultimately influence regulatory decisions that impact patient care.
Furthermore, the challenges faced in measuring these endpoints, particularly in the context of diverse regions like Latin America, highlight the necessity for robust data collection methods and clear communication strategies. Variability in data collection and participant adherence can introduce significant biases, underscoring the importance of establishing rigorous protocols to ensure reliable outcomes. The integration of biomarkers and surrogate endpoints also presents both opportunities and challenges, requiring thorough validation to accurately reflect clinical benefits.
As the landscape of medical research continues to evolve, particularly with the rise of personalized medicine, the role of well-defined clinical trial endpoints will remain pivotal. By fostering collaboration among stakeholders and investing in education and awareness, the clinical research community can better navigate the complexities of endpoint selection, ultimately contributing to the advancement of innovative therapies and improved patient outcomes. The future of medical research hinges on this meticulous approach, reinforcing the critical role that endpoints play in shaping the trajectory of clinical trials and healthcare advancements.
Frequently Asked Questions
What are clinical study objectives?
Clinical study objectives are specific events or results that a research aims to assess, which include various types of endpoints in clinical trials that determine the impacts of a treatment or intervention.
What are the types of endpoints in clinical trials?
Types of endpoints in clinical trials include survival rates, disease progression, and quality of life indicators.
Why is understanding types of endpoints in clinical trials important?
A comprehensive understanding of types of endpoints is crucial as they guide the study's structure and significantly impact regulatory decisions.
What unique challenges do Medtech companies face in Latin America?
Medtech companies in Latin America face challenges such as regulatory hurdles, professionalism issues, communication barriers, and fragmentation of resources.
How do these challenges affect clinical trials?
These challenges can impact the effectiveness of clinical trials, making it more difficult to track important endpoints like progression-free survival in patients.
What is an example of an endpoint critical for certain patient groups?
In patients with metastatic melanoma aged 12 years and older, tracking progression-free survival is a critical endpoint that informs treatment effectiveness.
How are patients with type 1 diabetes evaluated in clinical trials?
Patients with type 1 diabetes are evaluated through serum hemoglobin A1C levels, which serve as a traditional approval criterion for glucose-lowering therapies.
What role do partnerships play in Medtech innovations?
Partnerships, such as between Greenlight Guru and bioaccess™, are essential to speed up Medtech innovations and enhance research in the region.
What are the potential local economic effects of successful medical studies?
Successful medical studies can lead to job generation and advancements in healthcare, positively impacting local economies.
What must sponsors consider regarding the complexity of clinical trials?
Sponsors must carefully evaluate whether increases in complexity are necessary, balancing cost, speed, and intricacy in their studies.
How does media coverage influence clinical research in Latin America?
Media coverage helps increase awareness about research studies, which can assist in closing the knowledge gap regarding clinical trials.
What are the classifications of clinical study objectives?
Clinical study objectives are classified into primary, secondary, and surrogate goals.
What are primary goals in clinical trials?
Primary goals signify the main outcomes that a trial is specifically designed to measure, providing core evidence necessary to evaluate a treatment's efficacy.
What are secondary objectives in clinical trials?
Secondary objectives provide additional insights that can enhance the understanding of a treatment’s effects.
What are surrogate measures in clinical trials?
Surrogate measures are indirect indicators that aim to predict clinical outcomes, such as using a decrease in tumor size to infer possible improvements in overall survival rates.
Why is it important to understand the differences between primary and secondary objectives?
Understanding these differences is crucial for researchers to create effective investigations and interpret the types of endpoints in clinical trials accurately.
What does the case study on T-Lineage Acute Lymphoblastic Leukemia demonstrate?
The case study shows improved survival rates due to methotrexate randomization, highlighting the importance of comprehending primary and secondary objectives in clinical research.
What skill is essential for physicians regarding research studies?
The ability to effectively assess outcomes of research studies, particularly randomized controlled studies (RCT), is an essential skill for every physician.




