Introduction
In the realm of clinical trials, the definition and selection of study endpoints serve as the bedrock for evaluating treatment efficacy and safety. These endpoints, which can be classified into primary and secondary categories, not only guide the design of studies but also influence the interpretation of results and the regulatory approval process.
As the landscape of medical research evolves, particularly with the emergence of novel therapies and methodologies, the importance of clearly defined endpoints has never been more pronounced. This article delves into the intricacies of study endpoints, exploring their classifications, regulatory considerations, and the challenges faced in their measurement.
By understanding the critical role that endpoints play in clinical trials, stakeholders can better navigate the complexities of research and contribute to advancements in medical science.
Defining Study Endpoints: The Cornerstone of Clinical Trials
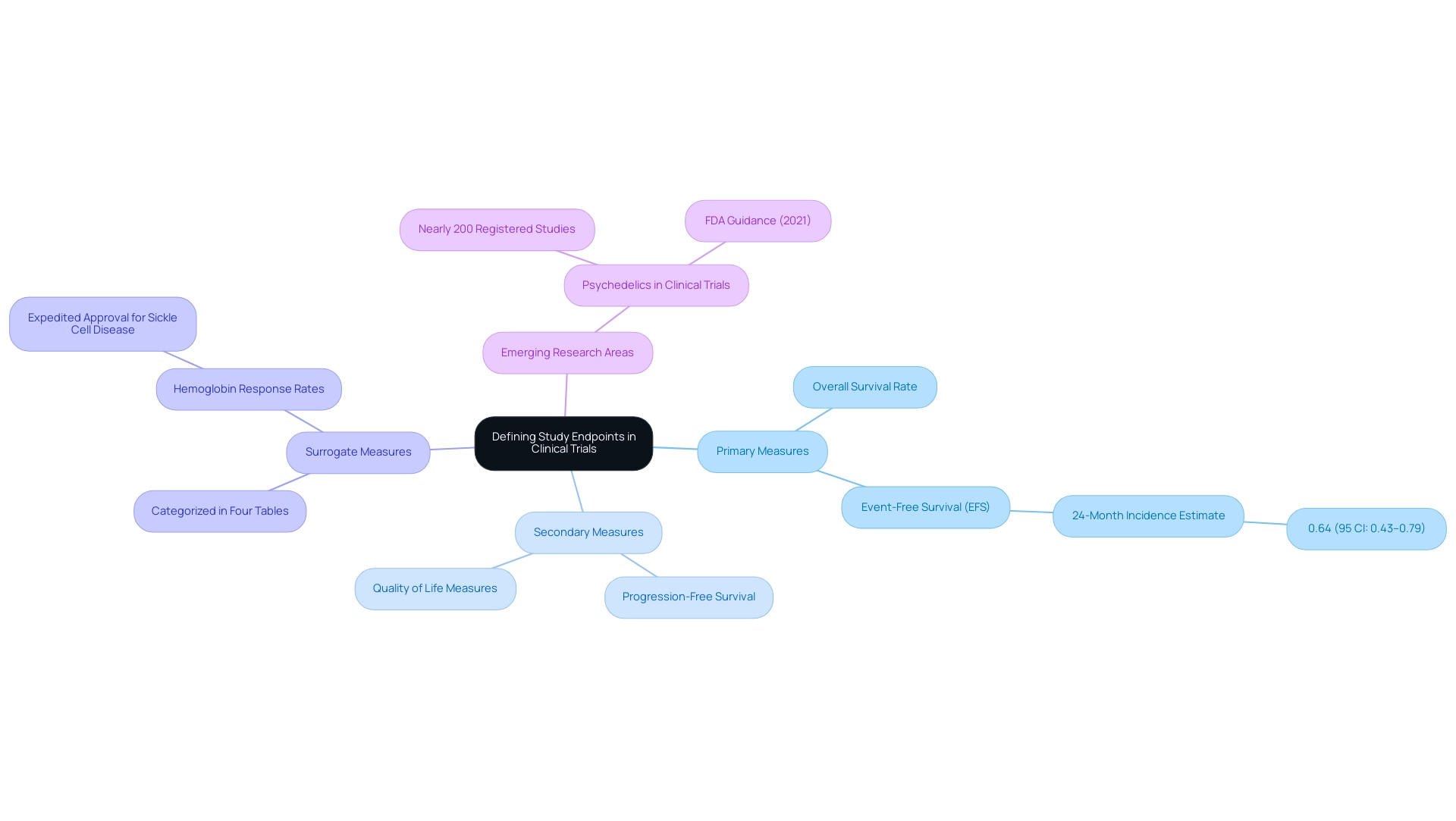
Study targets are crucial metrics that identify specific occurrences or results assessed to determine what is a study endpoint in evaluating the effectiveness of a treatment within research studies. They serve as critical benchmarks that help clarify what is a study endpoint in determining whether an intervention has achieved its intended therapeutic effect. Categories are classified into primary and secondary types, with primary measures representing the main focus of the study and secondary measures offering supplementary insight into the treatment's overall impact.
Grasping these markers is essential, as they influence the structure of medical studies and help define what is a study endpoint that ultimately determines the success or failure of a treatment. For instance, in a medical study evaluating a new cancer medication, the main goal might be the overall survival rate, while additional goals may include progression-free survival and quality of life measures. Recent estimates indicate that the 24-month incidence for event-free survival (EFS) using the cumulative incidence function method is 0.64, with a 95% confidence interval ranging from 0.43 to 0.79.
This statistic highlights the significance of clearly defined targets in assessing treatment effectiveness. Moreover, recent progress in clinical investigations, including almost 200 registered studies examining psychedelics, emphasize the evolving characteristics of research outcomes. With the FDA's initial draft guidance on psychedelics released in 2021, these developing fields of study offer chances to meet unmet medical requirements while emphasizing the essential function of measures in trial design and outcome evaluation.
Additionally, surrogate measures are categorized in four tables based on their use, providing a structured approach to understanding their application. As noted by W.T. Hwang, backed by the NIH, the changing environment of medical research highlights the importance of these outcomes.
Furthermore, the assessment of hemoglobin response rates for expedited approval of hemoglobin S polymerization inhibitors in individuals with sickle cell disease demonstrates the practical use of measures in present medical studies.
Exploring Different Types of Clinical Endpoints
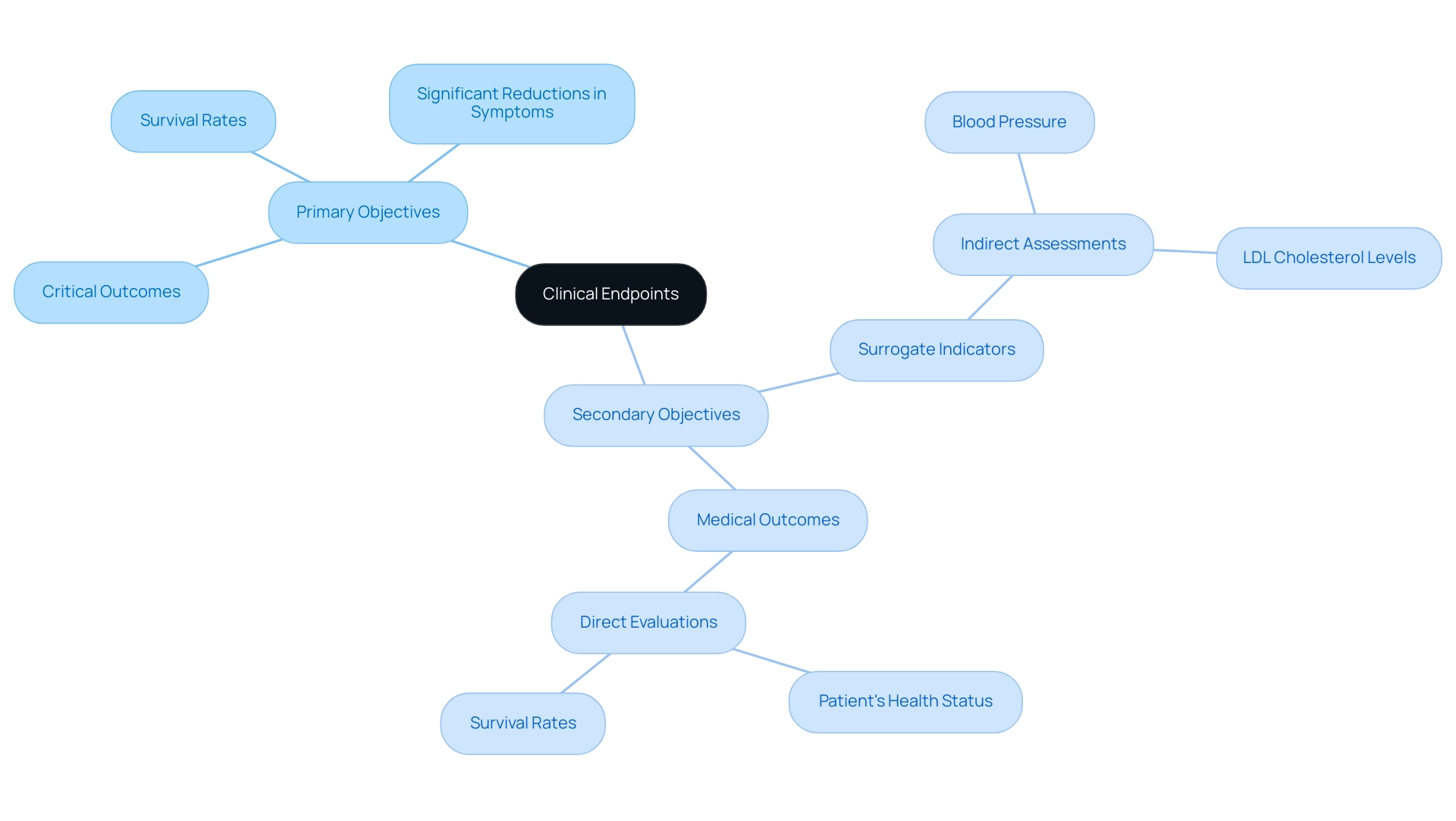
In research studies, a study endpoint includes clinical outcomes that are crucial for assessing the effectiveness of therapies and can be classified into primary and secondary outcomes.
-
Primary objectives represent the most critical outcomes that the study is designed to assess, such as survival rates or significant reductions in disease symptoms. For instance, in a recent medical trial, the second patient encountered an infection at roughly 325 days, emphasizing the significance of observing these outcomes carefully.
-
Secondary objectives provide additional insights, encompassing patient-reported outcomes or biomarkers that enhance the understanding of treatment effects. Moreover, targets are categorized into surrogate and observational measures, each fulfilling unique functions in research.
- Surrogate indicators are indirect assessments, such as blood pressure or LDL cholesterol levels, that are believed to forecast health results.
- In contrast, medical outcomes directly evaluate how a patient feels, operates, or survives. For example, in cardiovascular research, LDL cholesterol levels act as a surrogate measure, while actual instances of heart attacks or strokes are categorized as medical outcomes.
Grasping these distinctions is essential for researchers and stakeholders to accurately interpret clinical study results and understand what a study endpoint is to make informed treatment decisions.
Recent guidance from the FDA underscores the significance of careful examination when utilizing various measures, as highlighted by Dr. John Lawrence in a recent podcast discussion. Additionally, as noted by M. K. Wilson in The Lancet Oncology, understanding what a study endpoint is and bridging the gap between outcomes and endpoints is crucial for advancing cancer trials.
A case study titled 'Statistical Interaction in Subgroup Analysis' illustrates that statistical inspection of subgroups should not rely solely on P values but should include tests for statistical interaction to determine if patient groups differ significantly.
This nuanced understanding is vital for enhancing health outcomes and ensuring that research findings translate effectively into patient care.
The Importance of Clear Endpoint Definitions in Clinical Trials
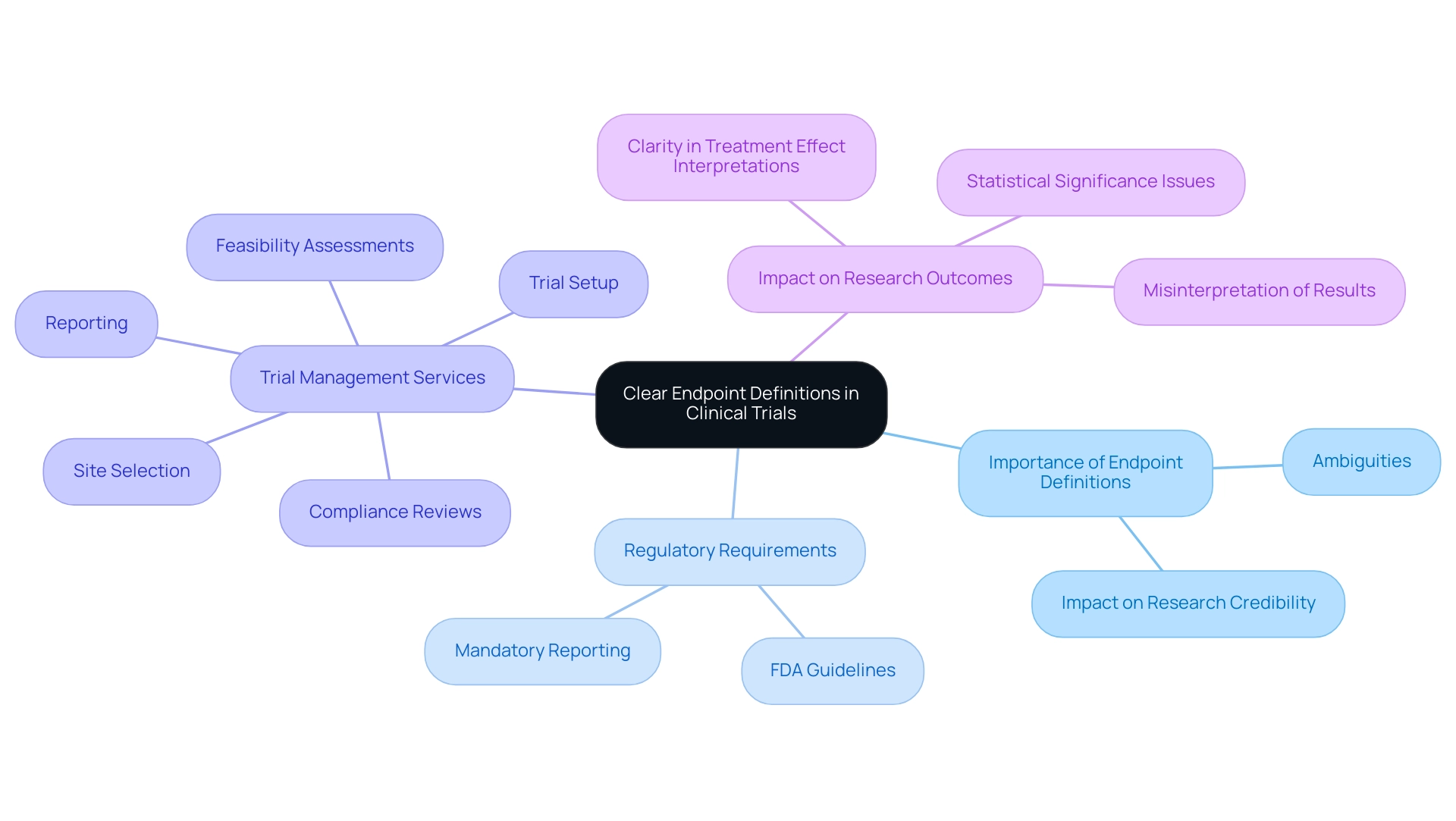
A precise description of limits is essential for the success of clinical studies. Understanding what is a study endpoint is crucial, as ambiguities or poorly defined endpoints can result in significant misinterpretations of results, undermining the credibility of the research and jeopardizing the potential for regulatory approval. For example, if a test fails to specify the criteria for 'improvement,' it may yield inconsistent results that are challenging to analyze and replicate.
Regulatory agencies, including the FDA, mandate precise definitions to accurately evaluate the efficacy and safety of new treatments. This is where our comprehensive clinical trial management services come into play, ensuring:
- Feasibility assessments
- Site selection
- Compliance reviews
- Trial setup
- Reporting (including trial status, inventory, and serious and non-serious adverse events)
Additionally, we offer comprehensive review and feedback on documents to comply with country requirements, which is essential for maintaining regulatory standards.
As highlighted in a systematic review, most non-inferiority RCTs reporting results that were not statistically significant for the primary outcomes showed distorted interpretation and inaccurate reporting. This emphasizes the need for clear outcome definitions, as they not only strengthen the reliability of the research but also improve communication among stakeholders, including researchers, sponsors, regulatory authorities, and help clarify what is a study endpoint. Moreover, statistics indicate that participants who examined abstracts with spin rated the research as less rigorous (p=0.034) and showed greater interest in reading the full-text articles (p=0.029).
Recent discussions on estimands indicate that mandatory reporting could improve clarity in treatment effect interpretations, addressing the limited practical application of estimands and emphasizing the need for clearer guidelines. The piece named 'Misleading Reporting in Statistically Not Significant Oncology Trials-Joining Efforts Toward Unbiased Results Interpretation' highlights the effects of unclear objectives in medical research. Ultimately, precise goal definitions are vital for enhancing medical knowledge, ensuring patient safety, and maximizing the beneficial influence of Medtech research on local economies, including job creation and healthcare enhancement.
Regulatory Considerations for Study Endpoints
Regulatory factors are essential in defining what is a study endpoint and specifying study goals in research studies. Organizations such as the FDA and EMA have created extensive guidelines that outline acceptable outcomes for different clinical study types. These guidelines are pivotal in ensuring that what is a study endpoint is both scientifically valid and clinically meaningful.
For example, in oncology research, overall survival is often considered what is a study endpoint, whereas chronic disease investigations may focus on quality of life metrics. Adherence to these regulatory stipulations is critical for the approval of new drugs and therapies; non-compliance can lead to significant delays in results submission, potentially extending up to two years if the sponsor certifies that the FDA has not yet approved the investigational product. Furthermore, the complexities of regulatory considerations are particularly evident in research involving vulnerable populations, such as children, where informed consent must be obtained from a legal representative.
This focus on adherence is reflected in the extensive management services we provide, which encompass:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Project setup with ethics committee authorization
- Import permits
- Continuous project management and reporting
As noted by the Department of Health & Human Services (HHS), it is essential that for research involving adult participants with mental illnesses or cognitive impairments, the ethics committee and investigators must be knowledgeable about the condition and any level of impairment likely to be present in the participant population. A comprehensive grasp of the regulatory environment, backed by our specialists including Ana Criado in Regulatory Affairs and Katherine Ruiz in medical device regulations, is vital for research professionals, allowing them to create effective projects that adhere to compliance standards and aid in successful drug development.

Challenges in Endpoint Selection and Measurement
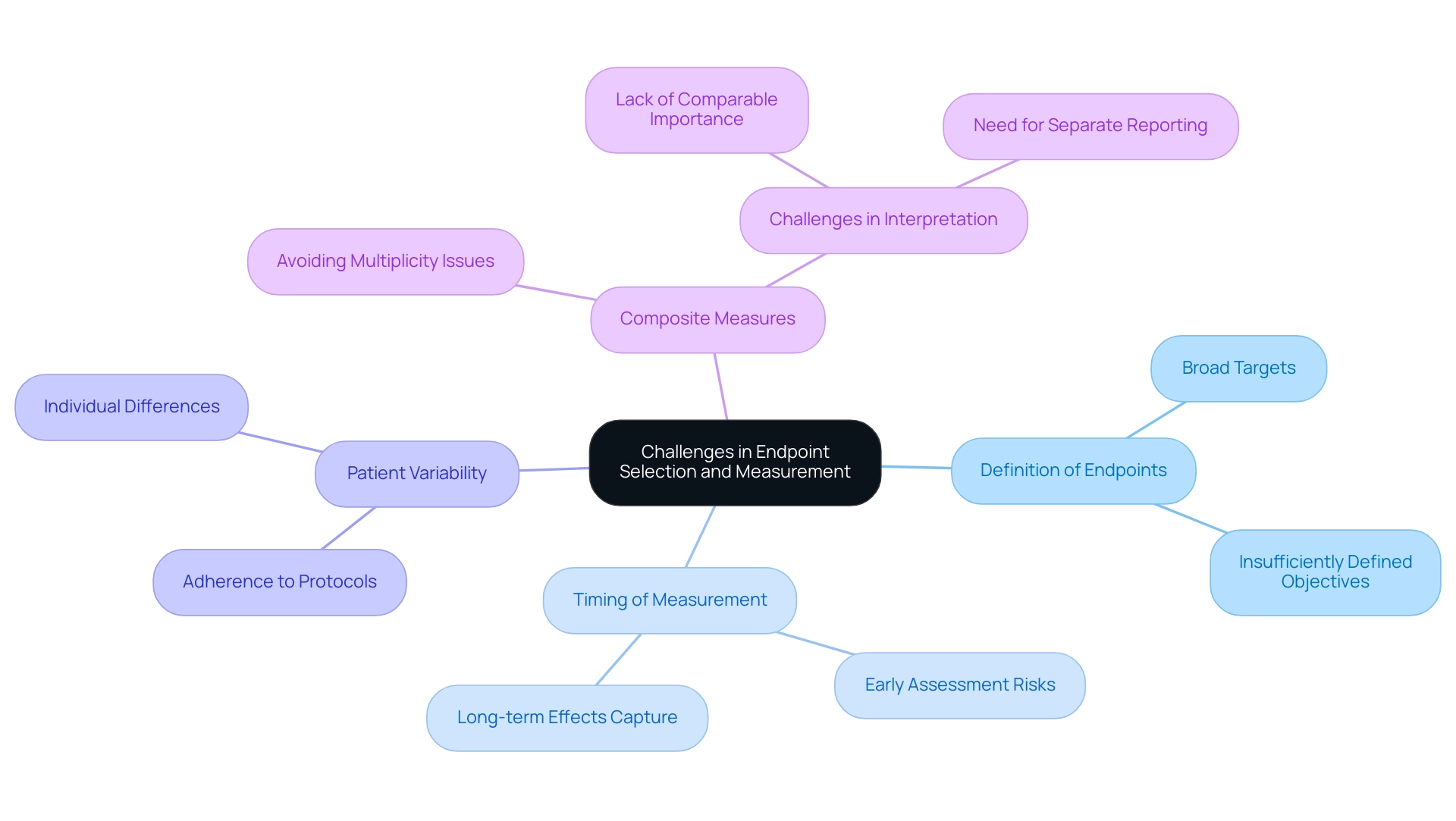
Choosing and assessing outcomes in clinical trials raises the question of what is a study endpoint, which is fraught with challenges that can significantly impact the validity of study results. A predominant concern is the selection of targets that may be overly broad or insufficiently defined, which can lead to variability in data collection and subsequent analysis. The timing of measurement is particularly critical; for instance, assessing a primary target too early may fail to capture the treatment's long-term effects, skewing the interpretation of efficacy.
Patient variability, including differences in adherence to treatment protocols, further complicates the consistency of outcome measurement. As emphasized in recent discussions, including a chapter in 'Rethinking Clinical Trials,' the complexities of selecting and defining objectives require careful consideration. Significantly, a case study on composite measures illustrates how combining indicators of multiple outcomes into a single metric can avoid multiplicity issues but also presents challenges in interpretation when components lack comparable importance.
As Robert Peter Gale observes, 'The intricacies of outcome measurement necessitate a nuanced comprehension to guarantee that clinical studies produce significant results.' To navigate these challenges effectively, researchers are encouraged to engage in meticulous planning and maintain open channels of communication with stakeholders throughout the study design phase. Employing validated measurement instruments and providing thorough training for data collectors are crucial strategies to improve the reliability of final data.
By proactively addressing these challenges, research trials can produce more robust and meaningful results, ultimately supporting better-informed healthcare decisions. Furthermore, the ongoing discussions around what is a study endpoint measurement challenges, as noted in the article in press since January 4, 2015, underscore the evolving nature of this critical aspect of clinical research.
Conclusion
Clear definitions and classifications of study endpoints are integral to the success of clinical trials, serving as the foundation for evaluating treatment efficacy and safety. The article underscores the critical role that both primary and secondary endpoints play in shaping study design, guiding statistical analyses, and influencing regulatory approvals. Ambiguities in endpoint definitions can lead to misinterpretations that undermine the credibility of research findings and potentially hinder the approval process for new treatments.
Regulatory agencies emphasize the need for precise endpoint definitions to ensure that clinical trials yield reliable and meaningful results. As clinical research evolves, particularly in emerging areas such as psychedelic therapies, stakeholders must remain vigilant in adhering to established guidelines while also adapting to new methodologies. The challenges associated with endpoint selection and measurement highlight the necessity for meticulous planning and robust communication among all parties involved.
Ultimately, the clarity and relevance of study endpoints not only enhance the quality of clinical trials but also contribute to advancing medical science and improving patient care. By prioritizing well-defined endpoints, researchers can better navigate the complexities of clinical trials, thereby fostering innovations that address unmet medical needs and improve health outcomes. The journey from endpoint selection to regulatory approval is a meticulous one, but with a steadfast commitment to clarity and precision, the potential for impactful medical advancements is vast.
Frequently Asked Questions
What are study targets in research studies?
Study targets are crucial metrics that identify specific occurrences or results assessed to determine study endpoints, which evaluate the effectiveness of a treatment.
What is the difference between primary and secondary study endpoints?
Primary endpoints represent the main focus of the study, such as survival rates, while secondary endpoints provide supplementary insights into the treatment's overall impact, including patient-reported outcomes or biomarkers.
Why are clearly defined study targets important?
Clearly defined study targets are essential as they influence the structure of medical studies and help determine the success or failure of a treatment.
Can you provide an example of study endpoints in a medical trial?
In a medical study evaluating a new cancer medication, the primary endpoint might be the overall survival rate, while secondary endpoints could include progression-free survival and quality of life measures.
What are surrogate measures, and how are they categorized?
Surrogate measures are indirect assessments believed to forecast health outcomes, categorized into four types based on their use in research.
What is the significance of the FDA's guidance on clinical trials?
The FDA's guidance emphasizes the careful examination of various measures in clinical trials, which is crucial for accurately interpreting study results and understanding treatment efficacy.
What role do surrogate indicators and medical outcomes play in research?
Surrogate indicators, such as blood pressure, are indirect measures that predict health results, while medical outcomes directly evaluate a patient's health, function, or survival.
How does understanding study endpoints improve patient care?
A nuanced understanding of study endpoints enhances health outcomes and ensures that research findings translate effectively into patient care, guiding informed treatment decisions.

