Introduction
In the realm of clinical research, the significance of well-defined clinical endpoints cannot be overstated. These measurable outcomes serve as critical benchmarks for evaluating the efficacy and safety of new treatments, guiding researchers in their quest to improve patient health.
As clinical trials become increasingly complex, particularly in fields like oncology, the challenge of selecting appropriate endpoints grows more pronounced. With evolving technologies and regulatory landscapes, understanding the nuances of primary and secondary endpoints, alongside emerging trends such as digital health data and patient-reported outcomes, is essential for researchers and stakeholders alike.
This article delves into the intricacies of clinical endpoints, examining their definitions, classifications, the challenges faced in their selection, and the regulatory frameworks that govern them, ultimately illuminating their pivotal role in the future of clinical trials.
Defining Clinical Endpoints: An Overview
In research trials, the criteria used to evaluate the effectiveness of a treatment or intervention are defined by what are clinical endpoints, which are specific measurable events or outcomes. These points function as crucial indicators of a treatment's effect on patient health, which raises the question of what are clinical endpoints for assessing both safety and efficacy of emerging therapies. Typical instances of medical outcomes include survival rates, disease advancement, and quality of life evaluations.
For medical researchers, understanding what are clinical endpoints is crucial, as these objectives greatly affect the planning, implementation, and analysis of medical studies. Recent analyses have revealed a growing complexity in study designs across various therapeutic areas, particularly in oncology, which has historically been the most intricate, with a steady increase in complexity from 2014 to 2020. This growing complexity underscores the necessity to understand what are clinical endpoints that can effectively assess treatment results amidst changing research environments.
Moreover, the obstacles encountered in medical studies, especially in Latin America, emphasize the significance of partnership between Medtech firms and local research organizations, such as bioaccess®. Their expertise in managing Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, and other crucial experiments can significantly enhance the efficiency and success rates of research in the region. The data indicating a considerable quantity of unsuccessful Alzheimer's medications in progress in the U.S. from 1998 to 2017 further emphasize the necessity for strong research management.
In the realm of new technologies, the FDA stresses a risk-focused strategy customized for AI/ML application, underscoring the significance of responsibility and clarity in the creation of medical outcomes, which is becoming more pertinent as research studies progress.
Types of Clinical Endpoints: Primary, Secondary, and Beyond
Research outcomes in studies are classified into primary and secondary goals, each fulfilling different roles that are essential for the study's analysis. The significant results that a clinical study is intended to evaluate, which directly reflect its primary aim, are often referred to as clinical endpoints. For example, in a study assessing a new cancer medication, overall survival is frequently used as a main goal, offering a clear indication of the treatment's effectiveness.
Conversely, secondary outcomes provide additional insights into the treatment's effects, which may include metrics like progression-free survival or quality of life assessments. Grasping these differences is essential for interpreting clinical endpoints in clinical research results and understanding their significance for healthcare.
A recent study named 'Characterization of Secondary Objectives in Oncology Studies' examined 280 late-phase oncology studies involving 244,576 individuals and discovered that only 22% of studies consistently listed all secondary objectives (SEOs) between ClinicalTrials.gov and their study protocols. The study revealed that while 69% of steps were published overall, the rates varied significantly by category, with patient-reported outcomes demonstrating notably lower publication rates. These findings emphasize the difficulties in precisely reporting outcomes, which is essential for improving the reliability of trial results and aiding informed decision-making in patient care.
Additionally, the FDA has released a three-page infographic and a podcast outlining guidance for drug sponsors, highlighting the need for caution in selection and reporting. As E.B. Ludmir, a fellow involved in the research, stated, 'This work was supported in part by Cancer Center Support (Core) grant P30CA016672 from the NCI to the University of Texas MD Anderson Cancer Center and by the Sabin Family Fellowship Foundation.
Challenges in Selecting Meaningful Clinical Endpoints
The choice of significant clinical outcomes raises the question of what are clinical endpoints, presenting a multifaceted challenge that requires careful consideration of various factors. Key to this process is ensuring what are clinical endpoints to individuals, as well as their capacity to be consistently assessed. Researchers must determine what are clinical endpoints in relation to the specific disease under investigation, the target population, and the study's objectives.
Furthermore, terminals should have adequate sensitivity to identify alterations in individual conditions over time. As emphasized in recent discussions, patient-reported outcomes are increasingly acknowledged as essential elements of clinical studies, with electronic capture methods offering improved accuracy and reliability. Poorly defined or irrelevant targets can lead to inconclusive results, raising questions about what are clinical endpoints, significantly hindering regulatory approval and ultimately affecting patient care.
Therefore, meticulous attention during the experiment design phase is paramount. As highlighted in WCG’s 2024 Clinical Research Site Challenges Report, to overcome their primary obstacles, research sites must adopt a culture of innovation and adaptability, particularly in understanding what are clinical endpoints when tackling selection challenges. The increasing tendency of pharmacy collaborations for participant recruitment, exemplified by Walgreens’ dedication to research studies, illustrates the significance of expanding access to possible candidates.
This trend not only improves recruitment efforts but also impacts the understanding of what are clinical endpoints. Expert opinions indicate that as we approach 2024, it will be crucial to define what are clinical endpoints in order to tackle persistent challenges in research, aligning study efforts with patient needs and expectations. Moreover, statistics show that inadequately defined medical outcomes, specifically what are clinical endpoints, are a primary reason for study failure, highlighting the essential requirement for accuracy in outcome selection.
Regulatory Landscape: Ensuring Compliance with Clinical Endpoints
The regulatory framework governing trial endpoints is primarily shaped by agencies such as the FDA and EMA, which delineate clear guidelines for defining and measuring these endpoints. Our extensive research management services support this framework by offering:
- Feasibility studies
- Site selection
- Compliance evaluations
- Detailed study setup processes, including obtaining necessary approvals from ethics committees and health ministries
- Management of import permits and the nationalization of [[investigational devices
Reporting](https://hoganlovells.com](https://rethinkingclinicaltrials.org/chapters/design/choosing-specifying-end-points-outcomes/choosing-and-specifying-endpoints-and-outcomes-introduction)/en/publications/fda-explains-how-to-use-secondary-endpoints-in-clinical-trials-to-show-efficacy) is a critical component of our services, encompassing study status updates and monitoring both serious and non-serious adverse events to ensure compliance and transparency. Adherence to these guidelines is paramount for the successful approval of new therapies, as it directly impacts the credibility of the clinical trial findings. Katherine Ruiz, a specialist in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, plays a crucial role in navigating the complex landscape of regulatory requirements, ensuring that chosen targets meet both scientific rigor and regulatory standards.
This alignment is vital for facilitating a more efficient review process. In light of ongoing discussions, the FDA's publication of a three-page infographic and podcast summarizing recent guidance reflects an effort to clarify these expectations. Key insights from these resources highlight the significance of understanding classification and adherence to evolving regulations.
Additionally, the overview of comments received on the draft ICH E9 (R1) addendum, published on 26/04/2018, highlights the ongoing dialogue surrounding regulatory standards. Furthermore, the case study titled 'Advancing the Use of Biomarkers and Pharmacogenomics in Drug Development' illustrates the practical implications of these guidelines, showcasing how integrating biomarkers can enhance drug efficacy and safety. As mentioned by regulatory expert Blake Wilson, 'If you have any questions about how to classify the outcomes in a trial or the capacity to draw conclusions from multiple outcomes in a trial, please do not hesitate to reach out to any of the authors of this alert or the Hogan Lovells attorney you typically collaborate with.'
This invitation highlights the importance of seeking clarity in categorization of final points, a crucial element in the compliance landscape.
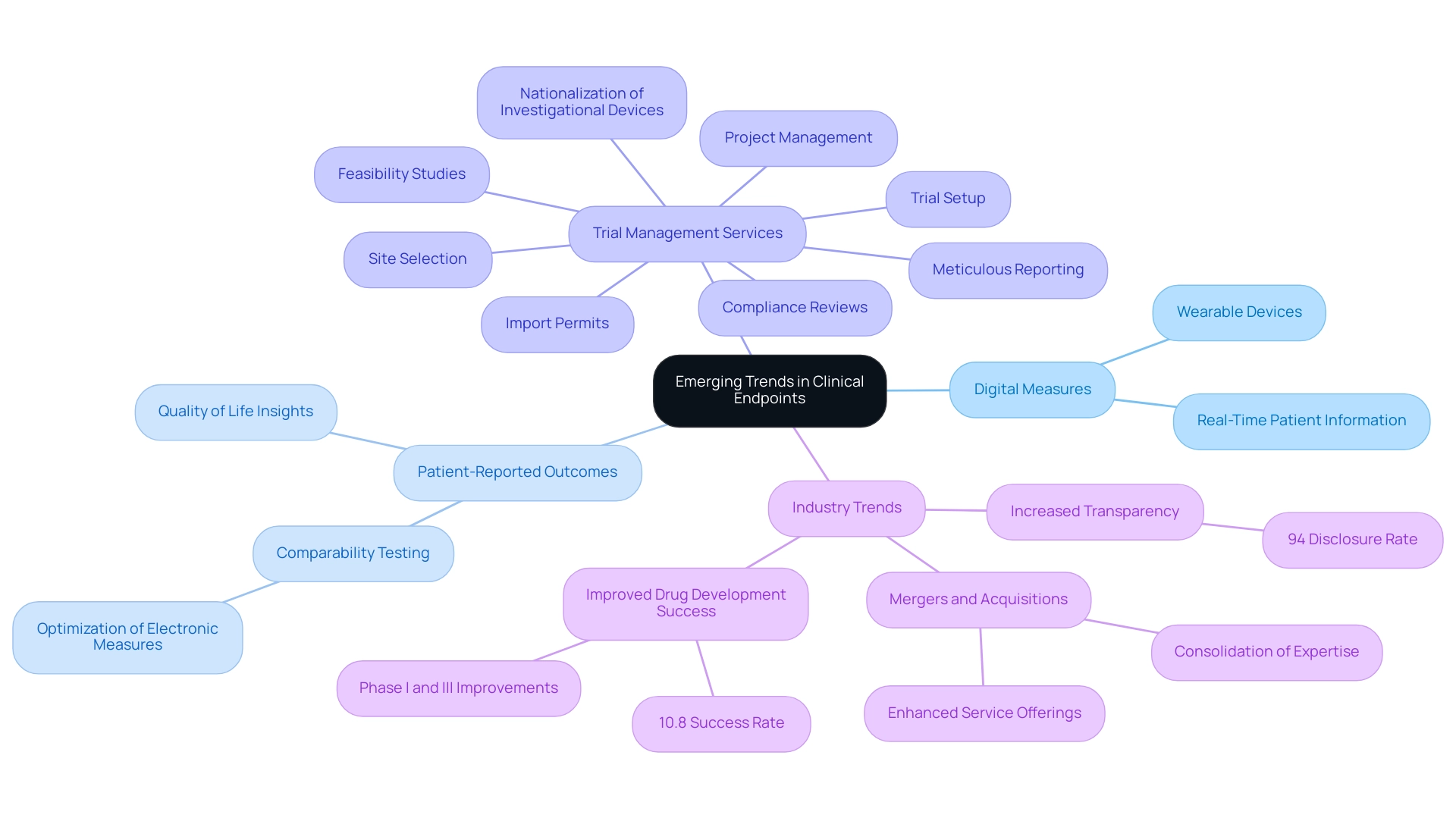
Emerging Trends: The Future of Clinical Endpoints in Trials
Recent progress in medical outcomes is increasingly centered on the incorporation of digital measures, which utilize technology to acquire real-time patient information. These innovations enable a more accurate and patient-centered assessment of outcomes, exemplified by the use of wearable devices that monitor physiological changes. The medical landscape is also witnessing a heightened focus on what are clinical endpoints, such as patient-reported outcomes (Pros), which provide valuable insights into the treatment's effects on quality of life.
As noted by Florence Mowlem, PhD, Vice President of Science for ObvioHealth:
I hope this can be a turning point for the industry with regard to comparability testing. We can stop having [comparability] conversations so frequently, and instead we can start talking about optimizing our electronic measures for all individuals.
Moreover, as of May 2023, a significant 94% of interventional research studies have disclosed their findings, demonstrating a dedication to openness and the changing nature of outcome evaluation.
Notably, the overall success rate for drug development pipelines increased to 10.8% in 2023, rebounding from a decade low, suggesting that enhancements in Phase I and Phase III studies are improving the overall significance of what are clinical endpoints in the evaluation of treatment effectiveness. Additionally, research by Rosenkranz et al. has investigated the influence of comorbidities on treatment outcomes in pulmonary arterial hypertension, emphasizing the significance of considering varied patient populations in outcome evaluations.
As these trends persist in influencing the future of medical studies, they promise to improve the understanding of what are clinical endpoints in evaluating treatment efficacy. This evolution is supported by comprehensive trial management services, encompassing:
- feasibility studies
- site selection
- compliance reviews
- trial setup
- import permits
- nationalization of investigational devices
- project management
- meticulous reporting
The recent merger of two clinical research firms in December 2023 further illustrates the evolving landscape of clinical research, emphasizing the need to adapt to new methodologies, such as digital endpoints and Pros, to drive global health improvement through international collaboration and innovation in Medtech.
Conclusion
The exploration of clinical endpoints reveals their fundamental role in the structure and success of clinical trials. Defined as measurable outcomes, these endpoints serve as critical markers for assessing the efficacy and safety of new treatments. The complexity of modern clinical trials, particularly in fields such as oncology, necessitates a nuanced understanding of both primary and secondary endpoints. The recent trend toward integrating patient-reported outcomes and digital health data further emphasizes the need for endpoints that resonate with patient experiences and outcomes.
Challenges in selecting meaningful clinical endpoints underscore the importance of relevance and measurement reliability. The engagement of stakeholders, including regulatory bodies like the FDA and EMA, is vital in ensuring compliance and enhancing the credibility of clinical trial findings. The regulatory landscape not only guides endpoint definition but also shapes the overall trial design, making it essential for researchers to remain informed of evolving standards.
Looking ahead, the integration of emerging technologies and the focus on patient-centric measures promise to redefine the future of clinical endpoints. The rise of digital endpoints and real-time data collection is set to enhance the accuracy and relevance of outcome assessments, contributing to more effective treatments. As the landscape of clinical research continues to evolve, the emphasis on robust, well-defined clinical endpoints will remain pivotal in driving advancements in patient care and therapeutic innovations.
Frequently Asked Questions
What are clinical endpoints?
Clinical endpoints are specific measurable events or outcomes used to evaluate the effectiveness of a treatment or intervention in research trials. They serve as crucial indicators of a treatment's effect on patient health.
Why are clinical endpoints important for medical researchers?
Understanding clinical endpoints is essential for medical researchers as they significantly influence the planning, implementation, and analysis of medical studies.
What are typical examples of clinical endpoints?
Typical examples of clinical endpoints include survival rates, disease advancement, and quality of life evaluations.
How have study designs evolved in oncology research?
Recent analyses indicate a growing complexity in study designs across various therapeutic areas, particularly in oncology, which has seen a steady increase in complexity from 2014 to 2020.
What challenges are faced in medical studies in Latin America?
The obstacles encountered in medical studies in Latin America highlight the importance of partnerships between Medtech firms and local research organizations to enhance research efficiency and success rates.
What role does the FDA play regarding new technologies in research?
The FDA emphasizes a risk-focused strategy tailored for AI/ML application, stressing the importance of responsibility and clarity in the creation of medical outcomes as research studies progress.
How are research outcomes classified in clinical studies?
Research outcomes in clinical studies are classified into primary and secondary goals. Primary goals directly reflect the study's main aim, while secondary goals provide additional insights into the treatment's effects.
Can you provide an example of a primary clinical endpoint?
In a study assessing a new cancer medication, overall survival is frequently used as a primary clinical endpoint, providing a clear indication of the treatment's effectiveness.
What did the recent study on secondary objectives in oncology studies reveal?
The study found that only 22% of late-phase oncology studies consistently listed all secondary objectives, highlighting difficulties in accurately reporting outcomes, which is essential for improving the reliability of trial results.
What resources has the FDA provided for drug sponsors regarding clinical endpoints?
The FDA has released guidance materials, including a three-page infographic and a podcast, emphasizing the need for caution in the selection and reporting of clinical endpoints.




