Introduction
In the realm of clinical research, the definition and selection of primary endpoints are paramount to the success of trials and the effectiveness of new treatments. These endpoints serve as the cornerstone for evaluating a treatment's efficacy, guiding regulatory approvals, and shaping patient care.
As the landscape of clinical trials evolves, particularly with the integration of advanced methodologies and technologies, understanding the intricacies surrounding primary endpoints becomes increasingly critical. From the distinction between composite and surrogate endpoints to the methodological considerations in their selection, the implications of these choices extend beyond the trial itself, influencing healthcare outcomes and economic growth.
This article delves into the multifaceted role of primary endpoints, highlighting their significance through case studies, best practices, and the latest trends in clinical research.
Defining Primary Endpoints: An Overview
Main objectives signify the precise results that a research study is carefully crafted to assess, which serve as the primary endpoint of a study to gauge a treatment or intervention’s efficacy. These points are typically established prior to the commencement of the study and play a pivotal role in assessing whether the study achieves its objectives. For instance, in studies assessing new cancer treatments, main outcomes may encompass overall survival rates or progression-free survival measures.
Grasping the meaning and consequences of main objectives is crucial, not only for precise interpretation of research outcomes but also for maneuvering through the regulatory approval framework. Recent progress in medical research, especially in 2024, highlights the importance of well-defined primary endpoints of a study, as they are essential for showcasing treatment effectiveness and obtaining regulatory approvals. Our comprehensive research management services encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Study set-up
- Start-up and approval processes
- Review and feedback on study documents
- Reporting of serious and non-serious adverse events
Having clearly defined goals is essential for reducing study complexity; a 10 percentage point rise in Complexity Score correlates with a 33% to 36% increase in overall study duration. Furthermore, as the FDA highlights, adopting a risk-oriented strategy customized for AI/ML application is essential in the changing environment of medical studies, requiring responsibility and openness in establishing goals to guarantee trustworthy results. The shift towards digital biomarkers in medical trials presents both risks and opportunities; when developed and adopted safely, these technologies can significantly benefit patients and researchers, ultimately enhancing the effectiveness of medical trials while also contributing to job creation, economic growth, and improved healthcare in local economies.
The Significance of Primary Endpoints in Clinical Research
The primary endpoint of a study plays a crucial role in medical research, greatly influencing the design, analysis, and interpretation of the study. They are crucial in determining the success of a treatment and often serve as the primary endpoint of a study, forming the basis for regulatory approvals. For example, when a medical trial shows a statistically significant advancement in the main goal compared to a control group, this result can lead to the treatment's authorization for therapeutic use.
The intervention effect size risk ratios for Scenario C—0.60, 0.55, and 0.50—demonstrate the potential effectiveness of treatments evaluated under such conditions. Moreover, key objectives provide vital insight for stakeholders, such as researchers, regulatory agencies, and patients, who depend on these metrics to comprehend the anticipated results of a medical intervention. Our extensive research study management services include essential components such as:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study setup—including the import permit and nationalization of investigational devices
- Project management
- Monitoring
These components ensure that all aspects align with regulatory standards like those from INVIMA, Colombia's National Food and Drug Surveillance Institute.
As observed by clinical researchers, the importance of the primary endpoint of a study cannot be exaggerated; it is essential to both the structure and the successful conclusion of clinical studies. Recent evaluations emphasize their significance, acting as essential references for study designers in choosing suitable main objectives, particularly the primary endpoint of a study, and for end-users analyzing study outcomes to guide practice and policy. Furthermore, JLW highlights the significance of main objectives in obtaining further funding, as shown by the Yale Clinical and Translational Science Award (UL1 TR001863).
Furthermore, case studies like the Prepare experiment show how primary objectives can affect regulatory choices, with results indicating that varP consistently surpassed both minP and bonfT approaches across all correlation values, thus improving treatment success rates in various therapeutic fields. This impact is not only crucial for advancing healthcare but also contributes to job creation and economic growth within local economies, fostering international collaboration in the Medtech sector.
Types of Primary Endpoints: Composite and Surrogate Explained
Main objectives in clinical studies are mainly classified into two principal types: composite and surrogate measures. Composite measures combine several individual outcomes into a singular metric, significantly enhancing the efficiency of trials by capturing a broader range of effects. For instance, in a cardiovascular study, a composite measure may encompass critical events such as death, myocardial infarction, and stroke, providing a comprehensive view of treatment efficacy.
Conversely, surrogate measures serve as indirect indicators that replace a direct clinical outcome, often utilized when direct assessment is unfeasible. A relevant example involves the use of blood pressure reduction as a substitute measure to predict a decrease in stroke risk. Moreover, individuals with osteoporosis need bone mineral density assessment, highlighting the significance of measurement in different situations.
Grasping the subtleties between these types is vital for correctly interpreting research results and their ensuing effects on patient care. As John H. Powers, MD, states, 'Correspondence and requests for reprints should be directed to …', highlighting the need for a clear comprehension of outcomes in medical studies. Recent discussions in the field have emphasized the difference between 'reasonably likely' and 'validated' surrogate measures, especially in the context of chronic myeloid leukemia therapies, highlighting the evolving landscape of measure utilization in research.
Moreover, a case study on type 1 diabetes mellitus illustrates the practical use of serum hemoglobin A1C as a conventional approval metric, emphasizing the importance of substitute measures in medical research.
Choosing the Right Primary Endpoint: Methodological Considerations
Choosing the suitable primary endpoint of a study in clinical trials requires a comprehensive review of various methodological factors, including the traits of the study population, the particular disease involved, and the anticipated treatment effects. It is crucial for researchers to select a primary endpoint of a study that is not only clinically relevant but also easily measurable. Furthermore, this point must meet regulatory expectations and be achievable within the constraints of the study's timeline and budget.
Best practices promote the involvement of various stakeholders—such as clinical experts, researchers, and patient representatives—in determining the primary endpoint of a study. This collaborative approach ensures that the selected target accurately reflects real-world patient outcomes and addresses their needs. As Dr. Nitin B Jain emphasizes,
Due to such heterogeneity, treatment benefit for an individual patient may deviate from the reported average effect size,
highlighting the need for measures that consider individual variability.
Furthermore, secondary measures can be utilized to demonstrate efficacy, providing a broader understanding of treatment effects. With 4053 students currently registered in the Project Management course, the significance of outcome selection in research education is emphasized, as it influences future study designs. The case study named 'Conclusion on Endpoints in Clinical Research' emphasizes this idea, demonstrating that meticulous selection of outcomes is crucial in improving the quality of medical studies, ultimately resulting in strong evidence that informs the creation of new therapies.
Case Studies: Successes and Failures in Meeting Primary Endpoints
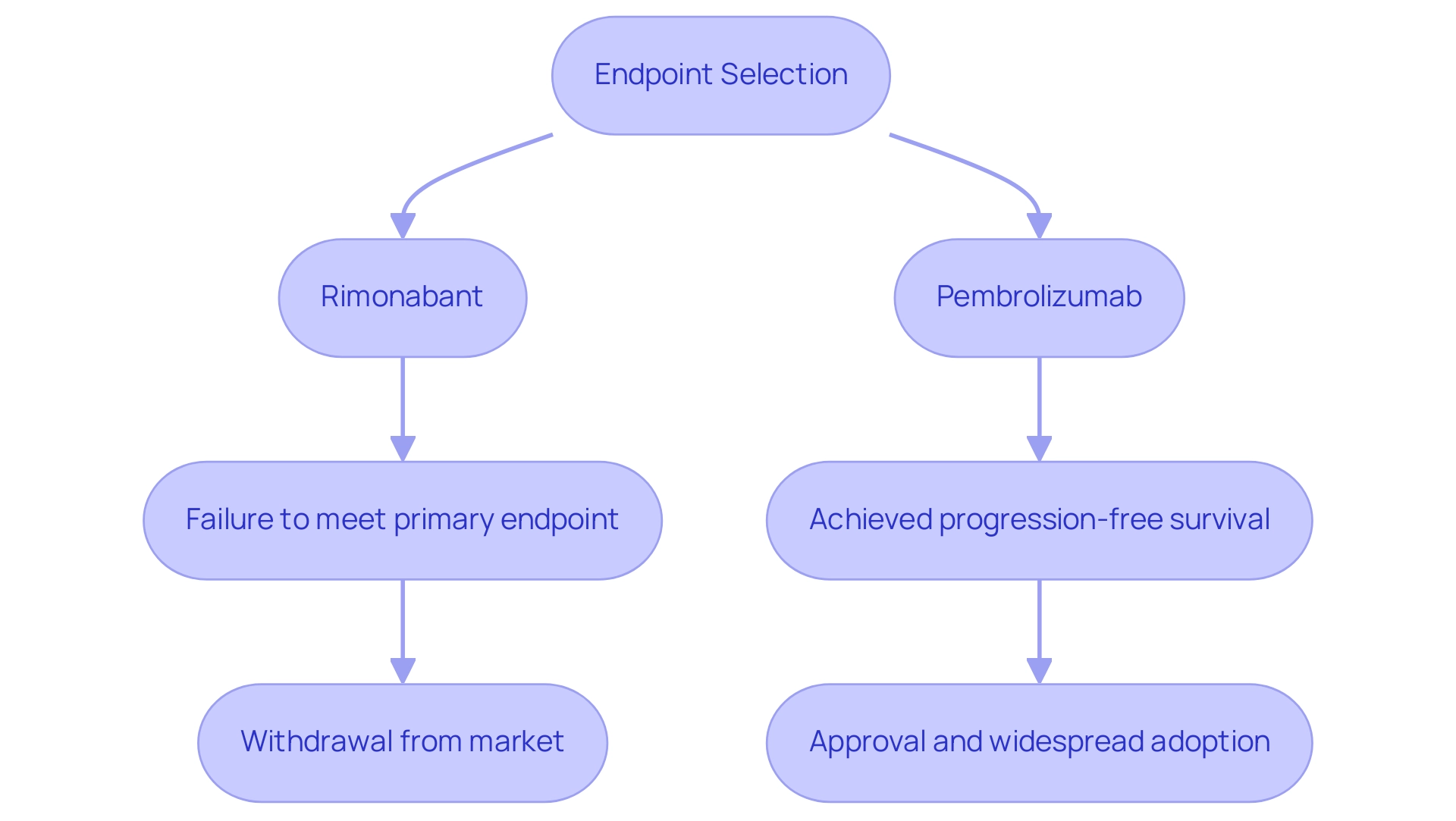
Case studies from research studies highlight the crucial function that the primary endpoint of a study serves in evaluating the effectiveness of interventions. A significant illustration is the study for Rimonabant, which sought to accomplish weight reduction as its main objective. Unfortunately, the test did not meet the primary endpoint of a study, resulting in the drug's withdrawal from the market due to safety concerns.
In contrast, the clinical trial for Pembrolizumab successfully achieved its main goal of progression-free survival, which not only facilitated its approval but also led to its widespread adoption in cancer treatment. Such contrasting outcomes emphasize the critical significance of choosing suitable primary endpoints of a study. Furthermore, the evolution of measures reflects the increasing complexity of patient responses, with a growing emphasis on individualized metrics that assess personalized risks and benefits.
This shift is vital, as it underscores the necessity for context-driven selection in clinical research, ultimately influencing the trajectory of drug development and patient care. As Green DJ et al. examined in their research on enrichment strategies in pediatric drug development, the meticulous choice of objectives can improve the significance and applicability of study outcomes.
Furthermore, as Robert Peter Gale observes, 'The choices regarding the primary endpoint of a study are crucial, as they not only define the success of a trial but also influence the future of therapeutic options available for patients.' This comprehensive approach to endpoint selection is essential for advancing clinical research.
Conclusion
The exploration of primary endpoints in clinical research reveals their foundational role in determining the success and efficacy of treatments. From defining primary endpoints to understanding their significance, the article elucidates how these measures guide the design, analysis, and interpretation of clinical trials. The differentiation between composite and surrogate endpoints highlights the complexities involved, emphasizing the need for precise definitions to ensure accurate outcomes.
Methodological considerations in selecting appropriate primary endpoints are critical, as they must align with regulatory expectations while reflecting real-world patient needs. Engaging diverse stakeholders in this selection process not only enhances the relevance of the chosen endpoints but also contributes to the overall quality of clinical trials. Case studies illustrate the tangible impact of primary endpoints on treatment approvals and market success, reinforcing the necessity of thoughtful endpoint selection.
In conclusion, the careful definition and selection of primary endpoints are vital for advancing clinical research and improving patient care. As the landscape of clinical trials continues to evolve, prioritizing well-defined endpoints will not only facilitate regulatory approvals but also foster innovation in treatment options. The implications of these choices extend beyond the trials themselves, ultimately influencing healthcare outcomes and economic growth within local economies.
Frequently Asked Questions
What are main objectives in a research study?
Main objectives are the precise results that a research study is designed to assess, serving as the primary endpoint to gauge the efficacy of a treatment or intervention.
Why are main objectives important in medical research?
They are crucial for interpreting research outcomes, navigating regulatory approval processes, and determining the success of treatments, as they form the basis for regulatory approvals.
How do main objectives affect the duration of a study?
Clearly defined goals help reduce study complexity; a 10 percentage point increase in Complexity Score can lead to a 33% to 36% increase in overall study duration.
What services are included in research management related to main objectives?
Services include feasibility studies, site selection, compliance reviews, study setup, start-up and approval processes, review and feedback on study documents, and reporting of adverse events.
What role do primary endpoints play in determining treatment success?
Primary endpoints greatly influence the design, analysis, and interpretation of a study, and significant advancements in these endpoints compared to a control group can lead to treatment authorization for therapeutic use.
How do primary objectives impact funding for research studies?
Main objectives are significant for obtaining further funding, as they guide study design and are essential references for stakeholders analyzing study outcomes.
What risks and opportunities do digital biomarkers present in medical trials?
When developed and adopted safely, digital biomarkers can enhance the effectiveness of medical trials and contribute to job creation, economic growth, and improved healthcare in local economies.
What is the significance of stakeholder insights related to key objectives?
Stakeholder insights are vital for researchers, regulatory agencies, and patients to understand the anticipated results of a medical intervention, guiding practice and policy.




