Introduction
The 510(k) submission process is a critical gateway for medical device manufacturers seeking to bring their innovations to market while ensuring compliance with the stringent standards set by the U.S. Food and Drug Administration (FDA). This regulatory pathway requires manufacturers to demonstrate that their devices are safe and effective by establishing substantial equivalence to existing, legally marketed devices.
The complexity of this process, which encompasses various submission types and the necessity for thorough documentation, can pose significant challenges. However, understanding the intricacies of the 510(k) process, from the importance of substantial equivalence to best practices for successful submissions, is essential for navigating this landscape effectively.
With insights from industry experts, this article delves into the key components of the 510(k) process, offering guidance for manufacturers aiming to achieve timely FDA clearance for their medical devices.
Understanding the 510(k) Submission Process
The 510k means that the submission process serves as a crucial regulatory route established by the U.S. Food and Drug Administration (FDA) for medical product manufacturers. This process allows companies to verify that their products are both safe and effective by showing substantial equivalence to a legally marketed item. Named after section 510(k) of the Food, Drug, and Cosmetic Act, 510k means that any product intended for human use must receive FDA clearance prior to marketing.
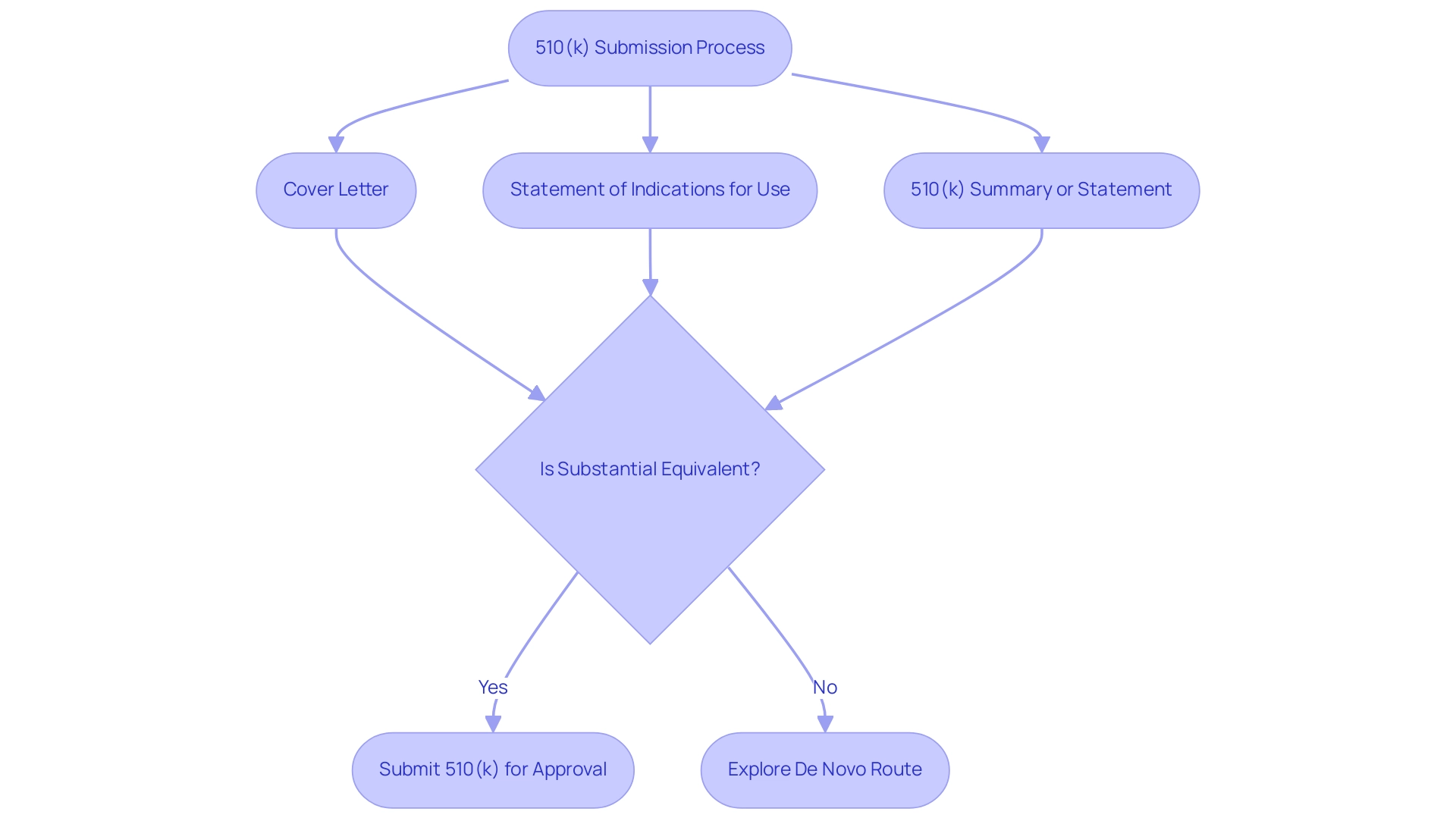
A comprehensive submission includes critical documents such as:
- A cover letter
- A statement of indications for use
- Either a 510(k) summary or statement
The FDA meticulously reviews these documents alongside detailed information about the apparatus, including its intended use, technological characteristics, and performance data, to ensure adherence to rigorous regulatory standards. Notably, manufacturers of components are not required to submit a 510k means unless the components are marketed as replacement parts.
For instance, the Annalise Enterprise CXR Triage Trauma instrument, developed by Annalise-AI Pty Ltd, successfully received approval on March 28, 2023, exemplifying the effective navigation of this submission process. Furthermore, if an item is classified as Class II and lacks a substantial equivalent, manufacturers may need to explore the de novo route, as noted by Qualio:
'If your product is definitely Class II and there’s really no substantial equivalent at all - which can happen if you have a really innovative medium-risk product - you’ll have to go down the de novo route.'
With experts like Ana Criado, Director of Regulatory Affairs, who has held significant roles at Colombia’s regulatory agency —INVIMA— and contributed to the development of regulatory frameworks, and Katherine Ruiz, a specialist in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, providing valuable insights and guidance, understanding what 510k means can significantly enhance navigating the complexities of the 510(k) process.
Ana's varied expertise also encompasses her position as the founder and CEO of Mahu Pharma, where she supervises the regulatory aspects of cannabis-based products, demonstrating her adaptability in the changing field of medical technology.
The Role of Substantial Equivalence in 510(k) Submissions
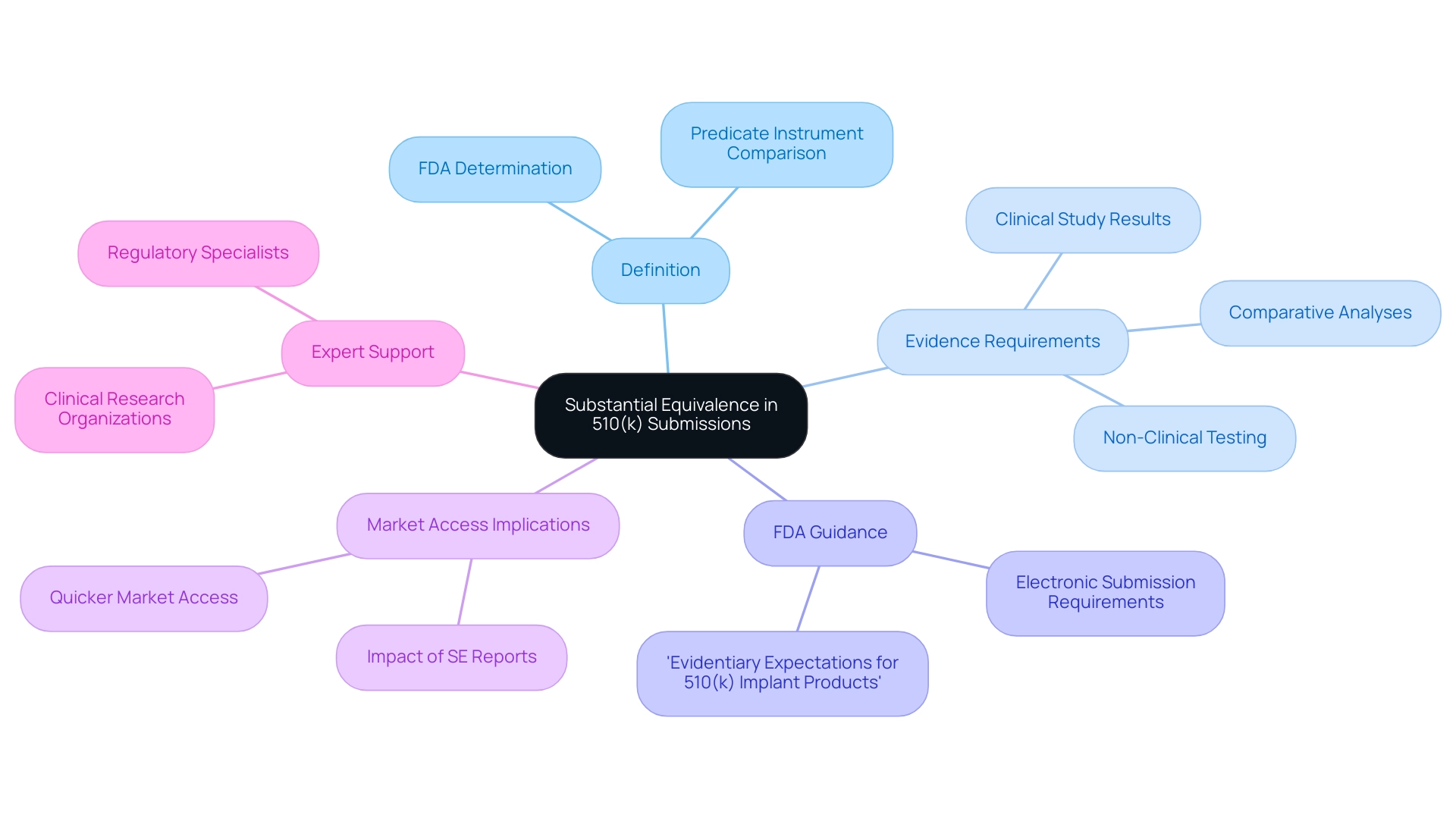
Substantial equivalence is a crucial determination made by the FDA, indicating that a new medical instrument is as safe and effective as an existing predicate instrument that is legally marketed. To substantiate this claim, manufacturers must present comprehensive evidence demonstrating that their product shares similar intended uses and technological characteristics with the predicate product. This evidence can encompass a variety of data, including clinical study results, non-clinical testing outcomes, and comparative analyses.
Recent FDA guidance, particularly the document titled 'Evidentiary Expectations for 510(k) Implant Products,' serves as a vital resource for manufacturers, outlining general recommendations relevant to all implant products requiring a 510(k) application. The importance of substantial equivalence is emphasized by the fact that approximately 90% of applications granted 510k means designation facilitate quicker market access for new devices. For example, the FDA's final rule requires that SE Reports be submitted electronically, highlighting the necessity of standardized forms to improve clarity and efficiency in the process.
Comprehending the nuances of substantial equivalence is crucial for manufacturers, as it directly affects their application strategies and the overall likelihood of securing FDA approval, which is what 510k means. Furthermore, with the evolving landscape of medical equipment regulations, partnering with experienced clinical research organizations like bioaccess® can provide invaluable support. Their expertise in managing early-feasibility studies, trial setup, site selection, compliance reviews, import permits, and project management ensures that manufacturers navigate the complexities of the approval process effectively.
Katherine Ruiz, a specialist in regulatory matters for medical products in Colombia, can assist companies in navigating the complexities of both local and global requirements, enhancing their likelihood of successful applications. As the FDA continues to refine its processes, staying informed about the latest decisions and requirements regarding substantial equivalence is imperative for success in the competitive medical equipment landscape.
Types of 510(k) Submissions: Traditional, Abbreviated, and Special
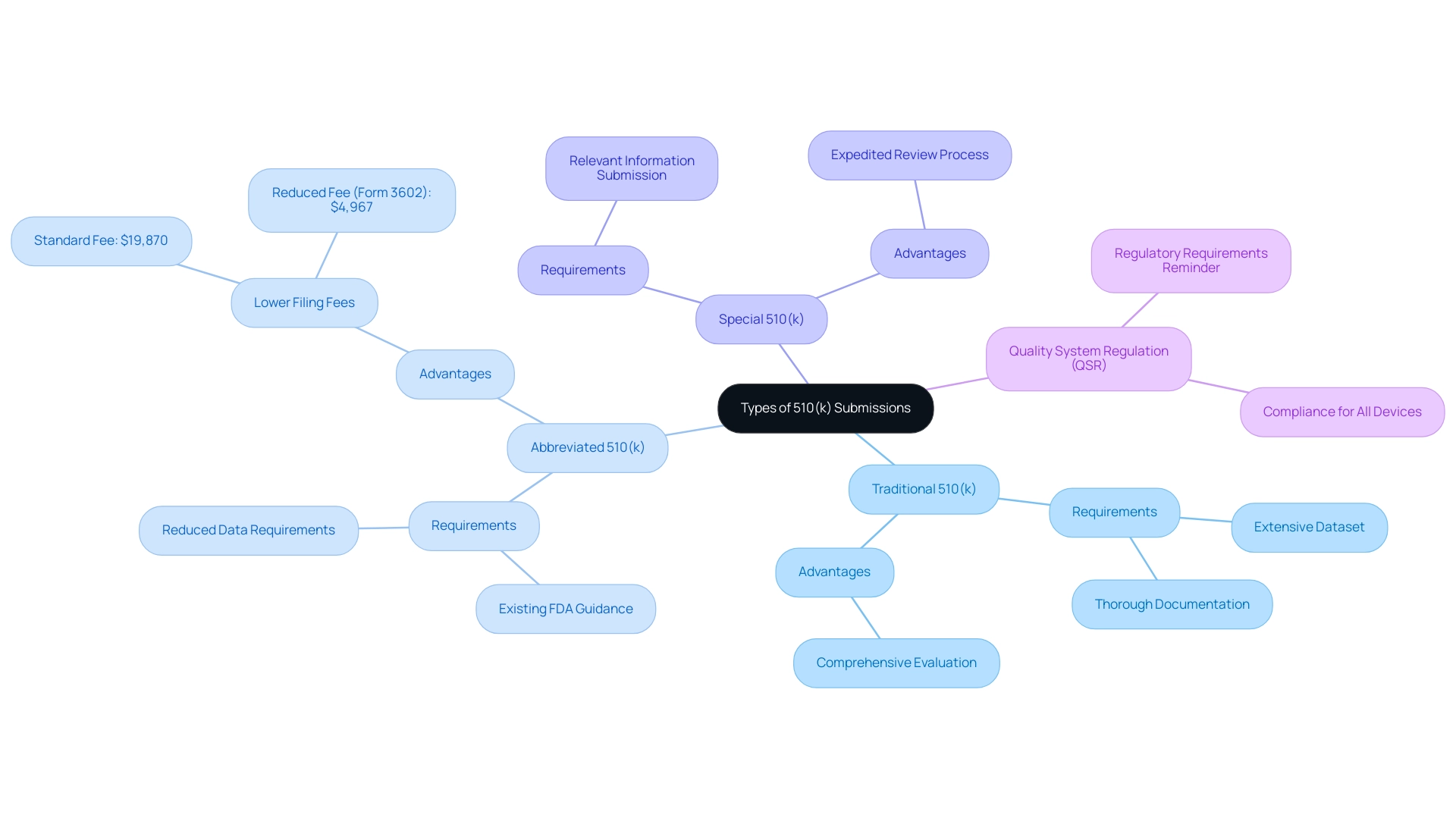
The 510k means that the application process consists of three main pathways: Traditional, Abbreviated, and Special, each designed for specific regulatory requirements and product classifications.
- Traditional 510(k): This submission type is the most frequently utilized, necessitating an extensive dataset to establish substantial equivalence. It necessitates thorough documentation, covering aspects such as specifications, labeling, and performance testing protocols, ensuring a comprehensive evaluation by the FDA.
- Abbreviated 510(k): Available for devices that fulfill designated criteria, this pathway allows manufacturers to demonstrate compliance through existing FDA guidance documents or recognized standards. By utilizing these resources, the data requirements are significantly reduced, simplifying the process of sending. Notably, small businesses can save significantly on 510(k) filing fees by completing FDA Form 3602, reducing the fee to $4,967 in 2023 compared to the standard fee of $19,870.
- Special 510(k): Designed for modifications to pre-cleared products, the Special 510(k) expedites the review process by permitting manufacturers to submit only the relevant information needed to confirm that the modification does not compromise the product's safety or effectiveness.
Understanding what 510k means is crucial for manufacturers as they navigate the regulatory landscape. As Ana Criado, our Director of Regulatory Affairs and Regulatory Affairs expert with over five years of experience at Colombia’s Regulatory agency —INVIMA— emphasizes, having a well-informed approach can significantly enhance compliance and operational efficiency. With her academic background as a biomedical engineering professor at Universidad Javeriana and Universidad de los Andes, along with her extensive experience consulting for global companies, Ana emphasizes the significance of choosing the most appropriate route for each specific item to enhance efficiency and compliance.
Furthermore, all medical instruments, including those exempt from what 510k means, must adhere to the Quality System Regulation (QSR) outlined in 21 CFR 820. This emphasizes the importance of compliance even for exempt devices, ensuring manufacturers are reminded of the regulatory requirements. Katherine Ruiz, an expert in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, also plays a vital role in navigating these complexities.
As Alex Pavlovic, a Quality and Compliance Expert, emphasizes, 'Fostering a culture of quality within manufacturing practices not only aids in meeting regulatory requirements but also drives growth and innovation in the life sciences sector.
Navigating Challenges in the 510(k) Submission Process
Navigating the 510(k) application process can pose significant challenges for manufacturers, especially because 510k means that inadequate data may not sufficiently substantiate substantial equivalence and could lead to misinterpretation of FDA requirements. These issues, combined with delays in acquiring essential testing results, can complicate the process of sending. Moreover, difficulties in communication with the FDA frequently result in extended review times.
Recent data indicates that six specialties exhibited significantly lower recall rates than the orthopedic reference category, emphasizing the critical need for precise documentation. To effectively navigate these hurdles, manufacturers should prioritize thorough pre-submission planning and engage with the FDA early in the process. Our comprehensive clinical trial management services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
provide vital support during this phase.
For instance, our compliance reviews ensure that all study documents meet the necessary regulatory standards, reducing the risk of misinterpretation by the FDA. Project management services facilitate smooth communication and coordination, addressing potential delays proactively. Employing document automation tools such as DocShifter can further streamline the preparation of filings, as 510k means managing complex documentation efficiently, resulting in quicker processes and enhanced organization of required documents.
Additionally, ensuring that all submitted data is comprehensive and well-organized is crucial. The FDA offers guidance documents to assist in deciding if a modification requires a new filing, as understanding what 510k means is essential for remaining updated with regulations. As Mike Drues notes,
Mistake #7 - Formatting Submission Incorrectly: Include page numbers and an eCopy.
Also, the size of the file matters and needs to be named according to conventions. By anticipating potential obstacles and following best practices, including acknowledging the limitations of adverse event reporting, such as our detailed reporting services that track study status and adverse events, manufacturers can significantly enhance their chances of a successful application.
Best Practices for Successful 510(k) Submissions
To enhance the likelihood of a successful 510(k) filing, manufacturers should implement several best practices:
-
Conduct Thorough Research: Familiarity with the regulatory requirements and specific guidelines relevant to your equipment type is crucial. The FDA received over 30 sets of comments on the Draft Report and Plan, highlighting the importance of comprehensive understanding.
-
Engage with the FDA Early: Initiating pre-submission meetings allows manufacturers to clarify expectations and receive valuable feedback on their proposal strategies. As emphasized by Katrina Rogers,
If prompt review of equipment proposals is essential to your business, you should contemplate contacting your Senator to convey your support for this program,
highlighting the significance of proactive communication.
-
Prepare Comprehensive Documentation: All data—including performance testing results and labeling—must be meticulously documented and presented in a clear, organized manner. The 'Evidentiary Expectations for 510(k) Implant Devices' serves as a primary resource for manufacturers to ensure they meet necessary documentation standards.
-
Utilize Expert Resources: Engaging regulatory consultants like Ana Criado, Director of Regulatory Affairs and founder of Mahu Pharma, who has extensive experience in biomedical engineering and regulatory compliance, can provide tailored guidance throughout the submission process. For instance, Ana's expertise in health economics and pharmacoeconomics allows her to advise manufacturers on cost-effective strategies for compliance, ensuring all documentation meets FDA standards.
-
Stay Updated on Regulations: Regularly reviewing updates in FDA policies and guidance is essential for maintaining compliance and aligning with current expectations. Ana's role as a consultant allows her to keep clients informed about the latest regulatory changes, which can impact their submissions.
-
Learn from Case Studies: For instance, preamendment products, which are those legally marketed before May 28, 1976, that have not been significantly altered, do not require a PMA application. These products are regarded as grandfathered and do not require a 510(k) as long as they retain the same intended use as when they were initially marketed. This example illustrates the importance of understanding device classification and the implications for the 510(k) process, a topic Ana often emphasizes in her consultations.
By adhering to these best practices, manufacturers can effectively navigate the intricacies of the 510(k) submission process, thereby increasing their chances of securing timely approvals.
Conclusion
The 510(k) submission process represents a vital step for medical device manufacturers aiming to achieve FDA clearance for their innovations. By demonstrating substantial equivalence to existing devices, manufacturers can facilitate quicker access to the market while ensuring compliance with rigorous safety and effectiveness standards. The article has outlined the critical components of this process, including the pivotal role of substantial equivalence, the various types of 510(k) submissions, and the challenges that manufacturers may face.
Understanding the nuances of substantial equivalence is essential for manufacturers, as it directly impacts their submission strategies and the likelihood of success. The exploration of the different submission types—Traditional, Abbreviated, and Special—provides valuable insights that can help companies select the most appropriate pathway for their devices. Furthermore, recognizing the common challenges in the 510(k) process, such as data inadequacies and communication hurdles with the FDA, underscores the importance of thorough preparation and proactive engagement.
By implementing best practices, including:
- Early FDA engagement
- Meticulous documentation
- Leveraging expert resources
manufacturers can significantly enhance their chances of successful submissions. Staying informed about evolving regulations and learning from industry experiences are crucial for navigating this complex landscape. Ultimately, a well-informed and strategic approach to the 510(k) submission process not only expedites market entry but also fosters innovation and growth within the medical device industry.
Frequently Asked Questions
What is the 510(k) submission process?
The 510(k) submission process is a regulatory route established by the U.S. Food and Drug Administration (FDA) for medical product manufacturers to verify that their products are safe and effective by demonstrating substantial equivalence to a legally marketed item.
What documents are required for a comprehensive 510(k) submission?
A comprehensive 510(k) submission includes a cover letter, a statement of indications for use, and either a 510(k) summary or statement.
What role does the FDA play in the 510(k) submission process?
The FDA reviews the submitted documents and detailed information about the medical apparatus, including its intended use, technological characteristics, and performance data, to ensure compliance with regulatory standards.
Are manufacturers of components required to submit a 510(k)?
Manufacturers of components are not required to submit a 510(k) unless the components are marketed as replacement parts.
Can you provide an example of a successful 510(k) submission?
An example is the Annalise Enterprise CXR Triage Trauma instrument, developed by Annalise-AI Pty Ltd, which received approval on March 28, 2023.
What should manufacturers do if their Class II product lacks a substantial equivalent?
If a Class II product lacks a substantial equivalent, manufacturers may need to explore the de novo route for approval.
Why is substantial equivalence important in the 510(k) process?
Substantial equivalence is crucial because it indicates that a new medical instrument is as safe and effective as an existing legally marketed product, facilitating quicker market access for new devices.
What types of evidence are required to demonstrate substantial equivalence?
Manufacturers must present evidence such as clinical study results, non-clinical testing outcomes, and comparative analyses to substantiate that their product shares similar intended uses and technological characteristics with a predicate product.
How does recent FDA guidance impact the 510(k) process?
Recent FDA guidance, particularly regarding implant products, provides recommendations and outlines evidence expectations, which are essential for manufacturers navigating the 510(k) application process.
How can manufacturers enhance their chances of securing FDA approval?
Manufacturers can enhance their chances of securing FDA approval by partnering with experienced clinical research organizations for support in managing the approval process and staying informed about evolving regulations.




